Reactions
Gasification is a partial oxidation process in which carbonaceous solids react with oxygen, enriched air or air to produce a CO/H2 mixture (syngas) according to the overall reaction:
CnHm + n/2 O2 → nCO + m/2 H2
The overall reaction can be summarized in several basic chemical reactions such as:
C + ½ O2 → CO ΔH = – 123.1 kJ/mol (1)
C + O2 → CO2 ΔH = – 123.1 kJ/mol (2)
C + CO2 → 2 CO ΔH = 159.7 kJ/mol (3)
C + H2O → CO + H2 ΔH = 118.9 kJ/mol (4)
In overall, gasification is a controlled combustion in O2 depleted atmosphere. In this case, most of the O2 fed to the gasifier is consumed in reactions (1) and (2). These reactions generate heat to increase temperatures at which chemical bonds are broken and gasification reactions (3) and (4) become favorable. If the gas is considered for a subsequent synthesis, the water-gas shift reaction (5), i.e.:
CO + H2O → H2 + CO2 ΔH = -40.9 kJ/mol (5)
also becomes important for adjusting the H2/CO ratio.
Otherwise, the primary objective is to maximize content of combustibles such as CO and H2. CH4 can also be formed at low gasification temperatures. Sulphur in the feed is converted mainly to H2S and small amount of COS. Traces of S2 and CS2 can also be formed. Most of the nitrogen in the feed is converted to N2. However, small amounts of HCN and NH3 are also formed. HCl is the main Cl-containing product formed during gasification[1].
Feedstock
There are a large number of different feedstock types for use in a gasifier, each with different characteristics, including size, shape, bulk density, moisture content, energy content, chemical composition, ash fusion characteristics, and homogeneity of all these properties. Coal and petroleum coke are used as primary feedstocks for many large gasification plants worldwide. Additionally, a variety of biomass and waste-derived feedstocks can be gasified, with wood pellets and chips, waste wood, plastics and aluminium, Municipal solid waste (MSW), Refuse-derived Fuel (RDF), agricultural and industrial wastes, sewage sludge, switch grass, discarded seed corn, corn stover and other crop residues all being used[2].
Mass Balance
Examples of mass balances from gasification processes are provided for coal and coke gasification respectively[1].
Processes
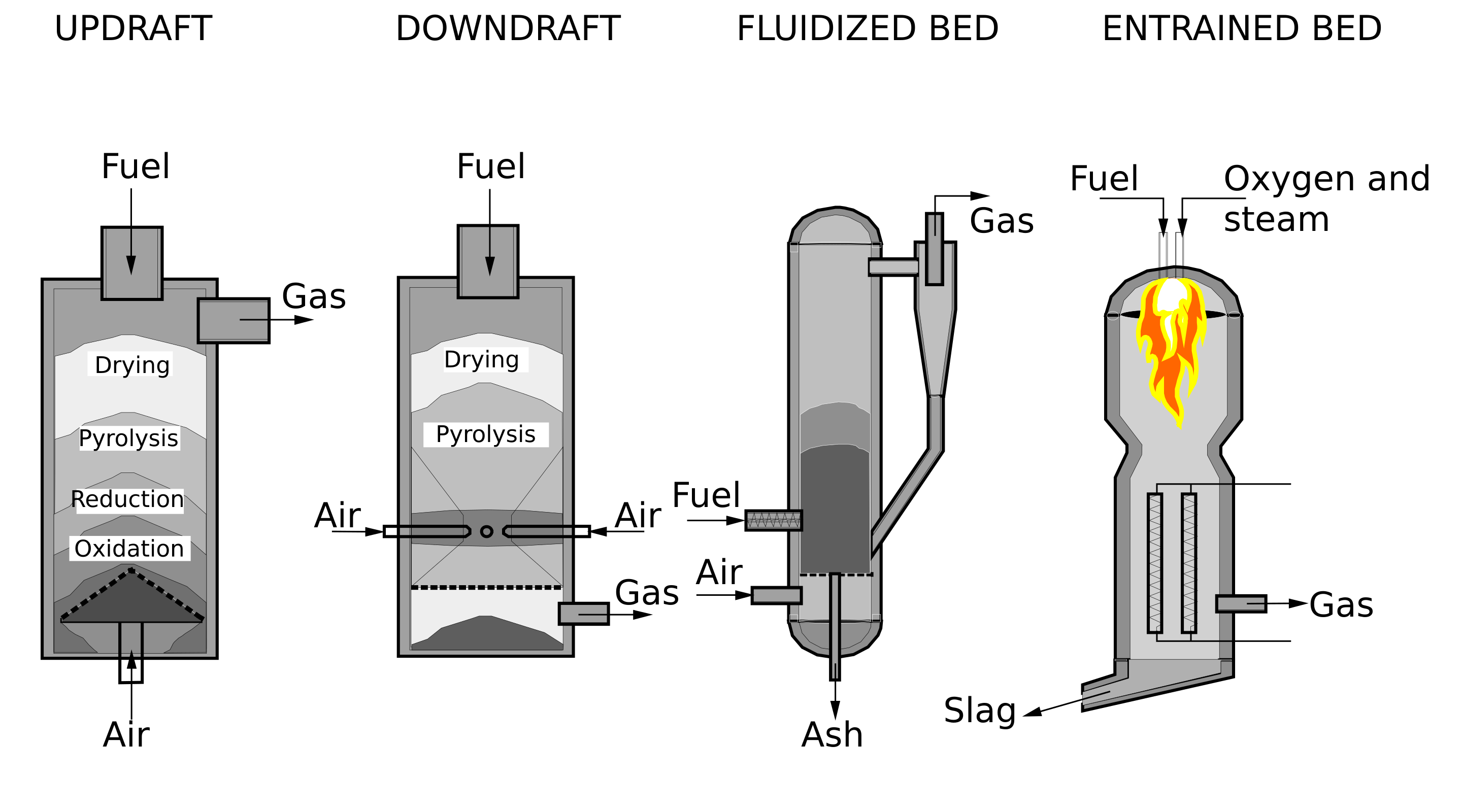
Several types of gasifiers are currently available for commercial use:
- Counter-Current Fixed Bed.
- Co-Current Fixed Bed.
- Fluidized Bed.
- Entrained Flow
- Plasma
- Free Radical.[2]
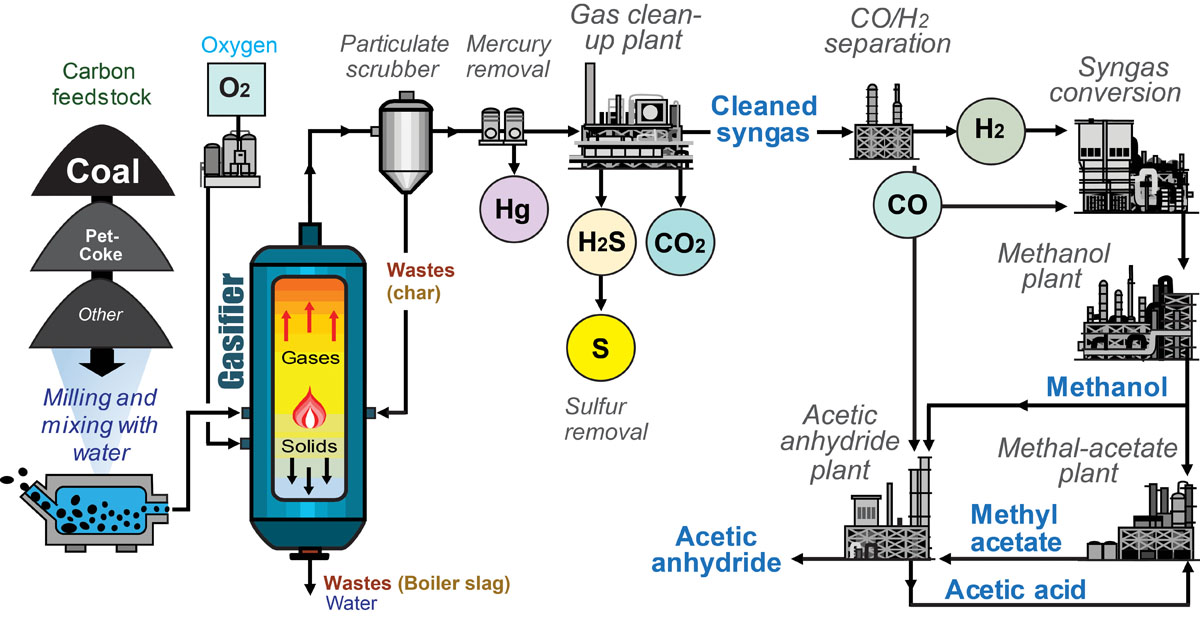
Coal Gasification Example
A good example of coal gasification used to produce chemicals from coal is the Eastman Chemical plant in Kingsport, Tennessee. At the Kingsport plant, coal or a mixture of coal and petcoke is gasified and particulate material is removed from the resulting syngas stream. Next, the syngas is purified through the removal of mercury (Hg), sulfuric acids (H2S), and carbon dioxide (CO2). The sulfuric acids are treated to form raw sulfur (S). Next, the cleaned syngas is separated into carbon monoxide (CO) and hydrogen (H2), which by themselves, are chemical products. Further treatment results in methanol (CH3OH), which is the source material for many important chemicals. One of those chemicals is methyl acetate, which is used in the production of acetic acid and acetic anhydride. The main function of the Kingsport plant is the production of acetic anhydride, which is used to make photographic film, synthetic textiles, and other products[3].
Global Gasification Technology Ranking by Capacity and Plants
Based on current commercial deployment (primarily in China, which dominates global gasification capacity), the global gasification technology ranking by installed capacity and number of plants is as follows[4][5]:
Table 1 - Leading Gasification Technologies (by Capacity/Market Share)
| Rank |
Technology
/Licensor |
Type |
Market
Share (Global) |
Key
Applications |
| 1 |
OMB
(ECUST) |
Entrained flow |
~32% |
Chemicals, fuels, power (China-focused) |
| 2 |
HT-L
(CECO) |
Entrained flow |
~22% |
Coal-to-olefins, ammonia (China) |
| 3 |
Air Products
Dry-Feed
(formerly Shell) |
Entrained flow |
~15% |
GTL, chemicals, IGCC (Global) |
| |
Air Products
Slurry-Feed
(formerly GE/Texaco) |
Entrained Flow |
~12% |
Hydrogen, power, chemicals (Global) |
| 4 |
'Gasifier'
Shenning/Sedin |
Entrained flow |
~8% |
Coal-to-liquids, methanol (China) |
| 5 |
Lurgi FDBD, BGL
Air Liquide
(formerly Lurgi) |
FDBD,
Moving
bed |
~6% |
SNG, fertilizers
Global) |
| 6 |
SFG®, GSP
Siemens
(formerly GSP) |
Entrained flow |
~5% |
Power, chemicals (EU/US/China) |
| 7 |
E-Gas Plus®
Lummus |
Entrained Flow |
~3% |
Refinery residues, petcoke (Global) |
Market Dynamics
- By companies:
- ECUST, CECO, Shenning/Sedin collectively hold >60% of China’s gasification capacity (30+ million Nm³/h syngas).
- Air Products holds the largest market share of about 27% at the global level combining gasification technologies acquired from Shell and GE/Texaco.
- New projects: 40+ gasifiers/year for coal-to-olefins and hydrogen.
- By Region:
- China: 37%
- North merica: 25%
- Europe: 18%
References
- Furimsky, Edward. (1999). Gasification in Petroleum Refinery of 21st Century. Oil & Gas Science and Technology-revue De L Institut Francais Du Petrole - OIL GAS SCI TECHNOL. 54. 597-618. 10.2516/ogst:1999051.
- Gasification, Wikipedia.
- Chemicals from Coal Gasification, University of Kentucky.
- Asiachem, 6th Nov 2017, China’s Coal Gasification Market Capacity Outlook 2017-2021.
- Coherent Market Insights, Feb 2025, GASIFICATION MARKET SIZE AND SHARE ANALYSIS - GROWTH TRENDS AND FORECASTS (2025 TO 2032).