Process Summary
Aromatics transalkylation and disproportionation (TDAP) is a significant process in modern aromatics complexes to produce valuable benzene and xylenes. The feed containing toluene, C9 aromatics, and hydrogen reacts over specialized catalysts (like zeolite) to produce benzene, toluene, and xylenes. The reaction takes place in fixed-bed reactors under controlled temperature conditions and requires hydrogen as a co-feed. The reactions result in decreased feedstock (toluene and C9 aromatics) while increasing products like benzene and xylenes with maximum xylene formation occuring with a feed containing a 50:50 ratio of toluene and C9 aromatics. This combined process is particularly important because neither xylenes nor benzene are produced in sufficient volumes from petroleum naphtha reforming to meet market demand.
TDAP Reactions
The TADP process is characterized by simultaneous transalkylation, disproportionation, dealkylation and isomerization of aromatic hydrocarbons such as toluene, and C9 aromatics such as trimethylbenzenes and ethyltoluenes.
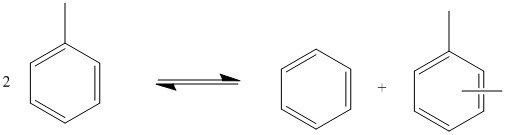
During the dispropornation reaction, the migration of an alkyl group from one aromatic molecule to another identical aromatic compound takes place. For example, toluene forms benzene and xylenes and trimethylbenzene forms xylenes and tetramethylbenzenes:
| |
Dispropornation reactions
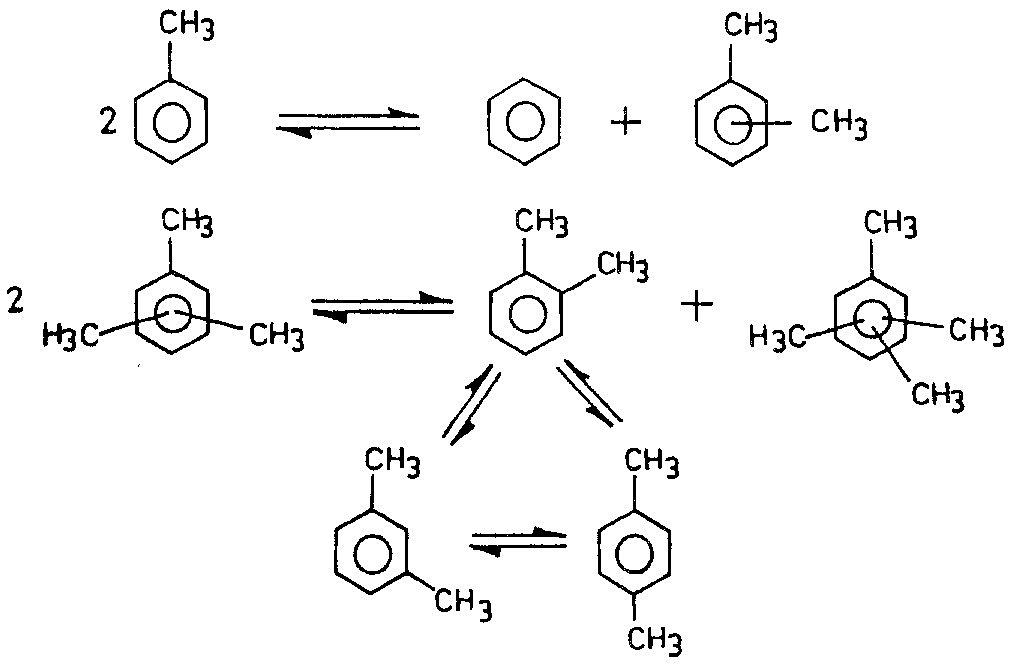 |
(1a)
(1b)
|
In case of an aromatic mixture conmprising methylethylbenzene, a dealkylation into toluene and ethylene takes place, whereas toluene is itsef disproportionated into benzene and xylene isomers.
| |
Dealkylation-Dispropornation reactions
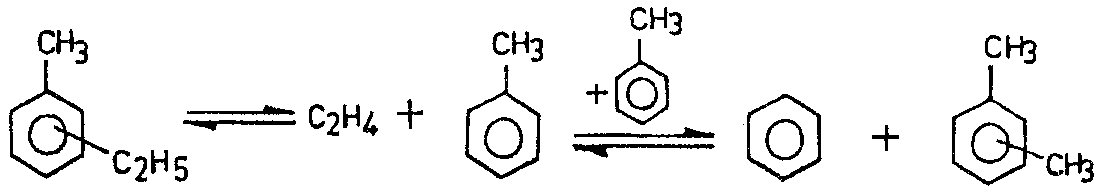 |
(2)
|
During reaction of arenes transalkylation the migration of alkyl group from the molecule of one aromatic compound to the molecule of a different hydrocarbon takes place. For example, toluene reacts with trimethylbenzene to form isomers of xylene:
| |
Transalkylation reactions |
|
| |
|
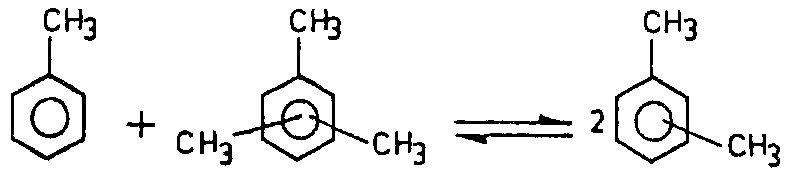 |
(3)
|
In addition, isomerization reactions are taking place, whereby positional isomers of the alkyl radicals of di- and tri-alkyl benzenes (xylenes, trimethylbenzene, ethyltoluene) are formed:
| |
Isomerization reactions |
|
| |
|
 |
(4a) |
| |
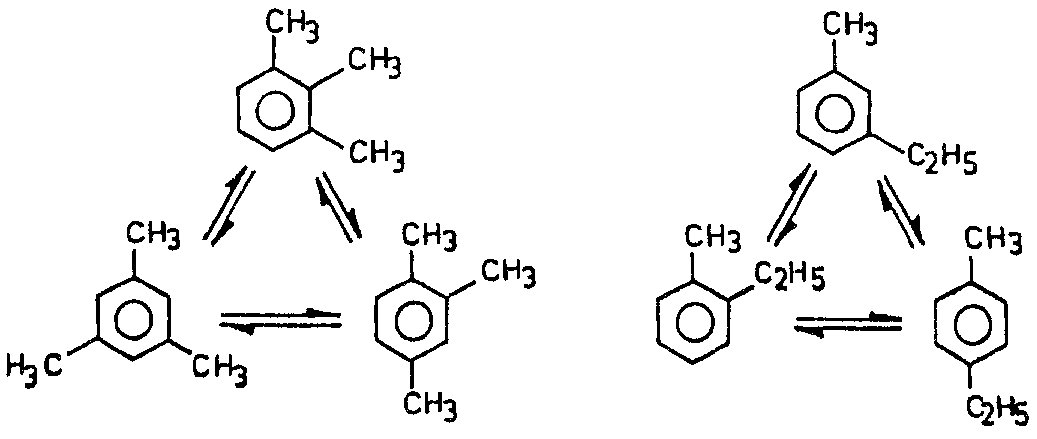 |
(4b)
|
Thermodynamic Dependencies
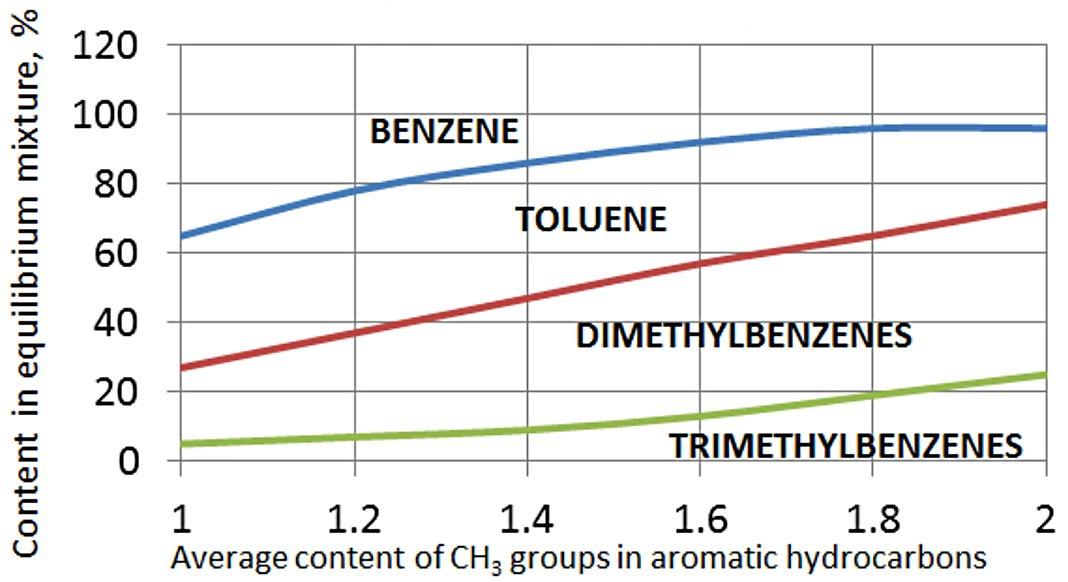
Thermodynamic dependencies of methylbenzenes disproportionation and transalkylation reactions have been studied by many authors. Fig. 1 shows the dependency of benzene, toluene, dimethyl- and trimethylbenzenes contents at the temperature of 477 °C. The equilibrium was stated to change insignificantly in the case of temperature variation in the range of 300-600 °C.
Figure 1 - Dependencies of benzene, toluene, dimethyl- and trimethylbenzenes contents in equilibrium mixture on aromatic hydrocarbon feed composition.
Presented data allow to calculate the equilibrium mixture composition at 477 °C, that is received trough disproportionation and transalkylation reactions as a function of concentrations in initial mixture of aromatic hydrocarbons – C7 and C9 methylbenzenes as shown in Table 1.
Table 1 - Data of equilibrium mixture composition as a function of feed composition
Equilibrium
mixture
composition,
% mol |
Feed Composition,
% mol Toluene/Trimethylbenzenes (TMBs) |
Toluene - 100%
TMBs - 0% |
Toluene - 70%
TMBs - 30% |
Toluene - 50%
TMBs - 50% |
| Benzene |
31.5 |
8.5 |
4.5 |
| Toluene |
42.0 |
37.0 |
21.0 |
| Xylene |
22.0 |
42.0 |
45.0 |
| Trimethylbenzene |
4.5 |
12.5 |
29.5 |
Process Flow Diagram
A typical TDAP process scheme is presented in Fig. 2, as originaly developed by Toray Industries in the early 1970s.
Figure 2 - Main process flow diagram of disproportionation and transalkylation unit
|
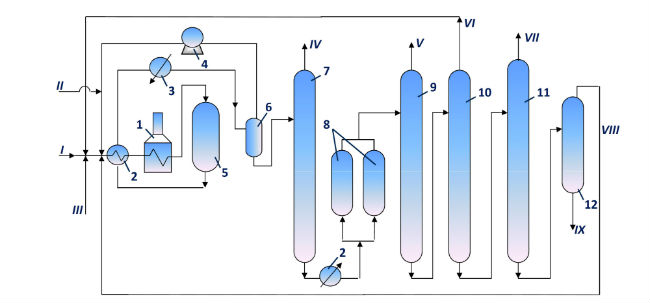 Legend: Legend:
- Equipment: 1. tube furnace; 2. heat exchangers; 3. cooler; 4. hydrogen gas recycle compressor; 5. reactor; 6. HP gas separator; 7. Stabilizer; 8. clay treatment adsorbers; 10. benzene column; 11 toluene column; 12. xylene column; 13. trimethylbenzene column.
- Product streams: I. toluene feed; II. hydrogen feed; III. C9 aromatic hydrocarbons feed; IV. stabilization products; V. benzene; VI. toluene recycle; VII. xylene; VIII. C9 aromatic hydrocarbons recycle; IX. aromatic hydrocarbons C10+.
|
This processing scheme may be used depending on the requirements for aromatic hydrocarbons, and also toluene and trimethylbenzenes (C9H12) resources using isomerization, disproportionation and transalkylation processes over zeolite catalysts without noble metal. The hydrodealkylation process is also possible to apply.
Process Details
Original feed – toluene – is mixed with recycle hydrogen gas, then it is heated in furnace and is fed to reactor. Reaction products are separated in HP gas separator. Gas phase goes to recycle compressor suction, and liquid phase is stabilized and treated with clay. Further the products are rectified to recover benzene, toluene, xylenes, and trimethylbenzenes. Benzene and xylene recovered from the liquid products are commercial products. Toluene and trimethylbenzenes are mixed with the feed. In order to increase xylene production, a mixture of C9 aromatic hydrocarbons produced by catalytic reforming may be added to the feed.
Process operating conditions are the following: 3 MPa (30 kgf/cm2); 400-450 °C; H2:HC, mole/mole 6-10:1; space velocity about 1.0 h-1; H2 content in recycle H2 gas; not less than 70 mol. %.
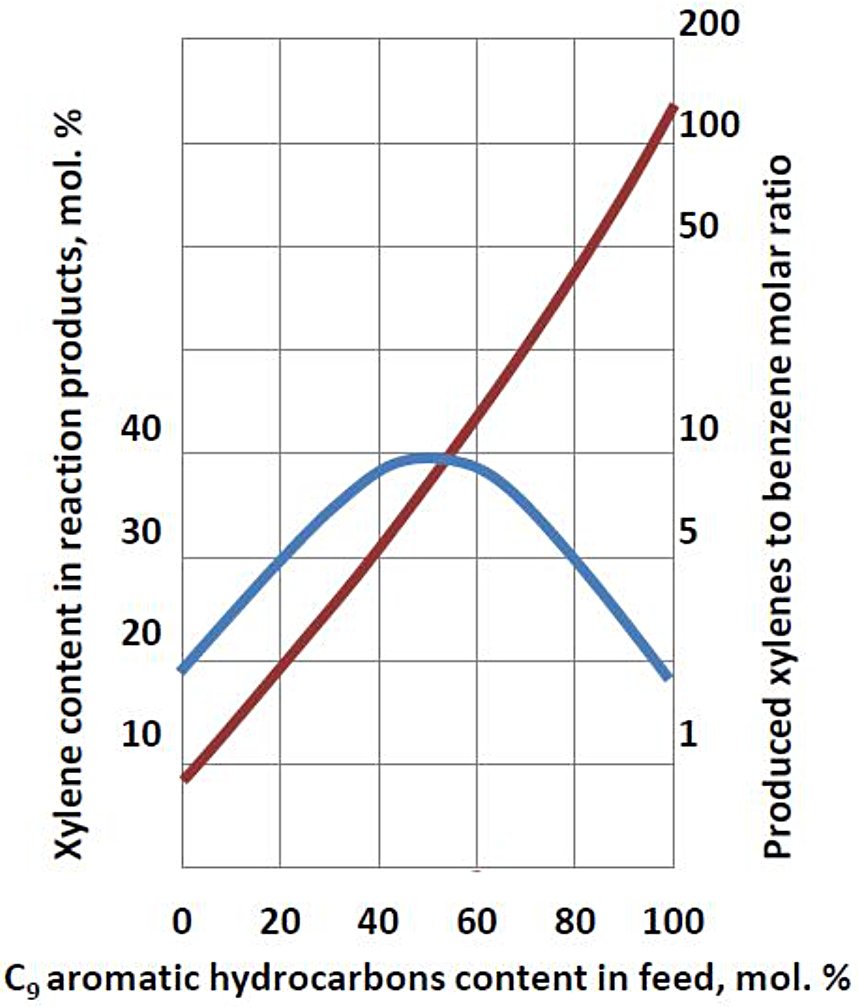
The unit is able to produce benzene and xylenes in the ratio from 0.8 : 1 to 1 : 10, subject to C9 aromatic hydrocarbons content in the feed (Fig. 3). If C9 aromatic hydrocarbons content in feed equals to 4 mol.%, then benzene/xylene ratio is 1. Maximum xylene content in reaction product is observed at 50 mol.% of C9 aromatic hydrocarbons content in the feed.
Figure 3 - Produced benzene/xylene molar ratio vs. molar content of C9 aromatic hydrocarbons in transalkylation feed.
Material Balances
Approximate material balances (in % wt.) of disproportionation and transalkylation processes with recycle of unreacted C7 and C9 aromatic hydrocarbons are shown in Table 2.
Table 2 - Approximate Mass Balances (in % wt.) of disproportionation and transalkylation processes with recycle of unreacted C7 and C9 aromatic hydrocarbons
| Weight % |
Reaction |
Dispropornation |
Transalkylation |
| Feed |
toluene |
100.0 |
75.0 |
| trimethylbenzenes |
- |
25.0 |
| hydrogen |
0.4 |
0.4 |
| TOTAL |
100.4 |
100.4 |
| Products |
benzene |
41.4 |
25.2 |
| xylene |
56.1 |
71.6 |
| C9+ aromatic hydrocarbons |
1.0 |
1.7 |
| Hydrocarbon gas, incl. losses |
1.9 |
1.9 |
| TOTAL |
100.4 |
100.4 |
References
- SIE NEFTEHIM, LLC, Aromatics Transalkylation and references therein. (accessed 3rd Jan 2024)
- Chen Qingling et al. CN12688405A. Priority date 30th Mar 1990. Toluene and C9 aromatics disproportionation and transalkylation catalyst. Assigned to: Sinopec Shanghai Research Institute of Petrochemical Technology,China Petrochemical Corp. Status: Application granted, Expired - Lifetime.
- Jeffrey T. Miller et al. CA2553514C. Priority date: 16th Nov 2024. Method of converting C9 aromatics - comprising mixtures to xylene isomers. Assigned to: BP Corp North America Inc. Status: Application granted, Expired - Fee Related.
- Das, J., Bhat, Y. S., & Halgeri, A. B. (1994). Transalkylation and disproportionation of toluene and C9 aromatics over zeolite beta. Catalysis Letters, 23(1-2), 161–168.
- Wikipedia. Dispropornation of toluene.