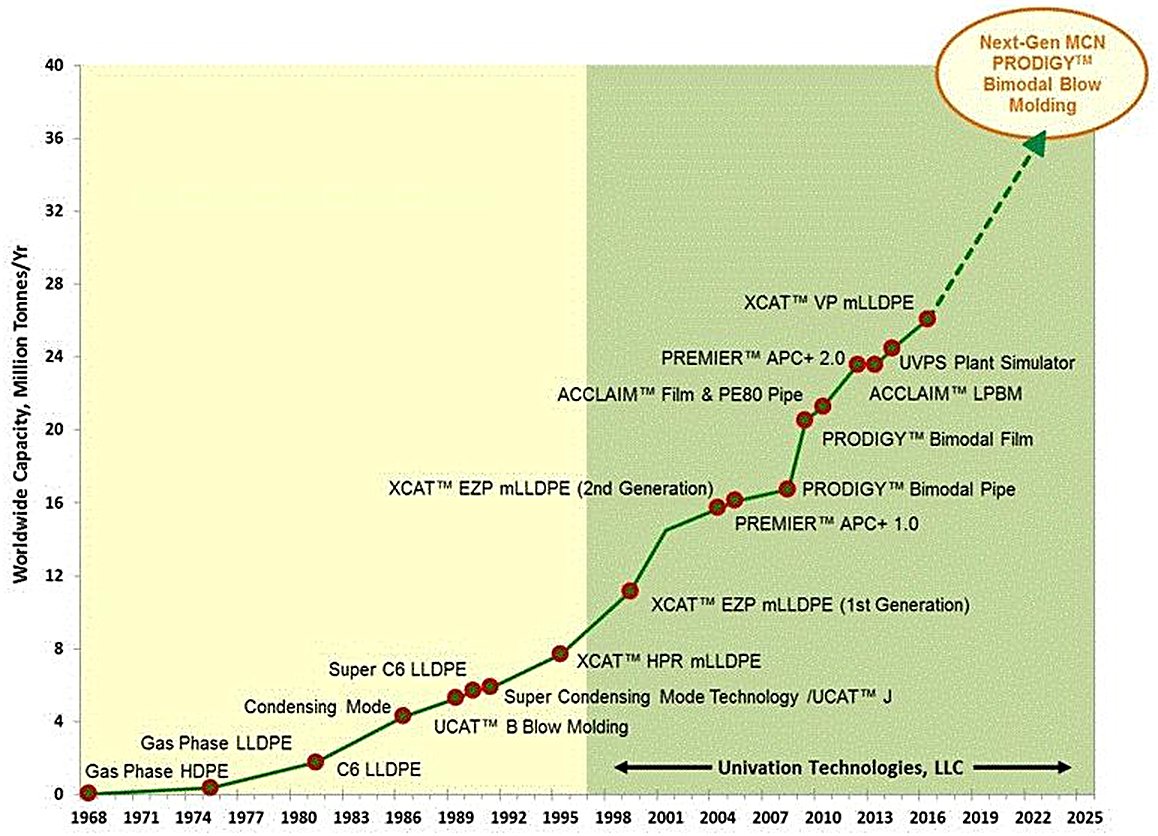
Technology History
The UNIPOL™ PE Process originated in the late 1960s, when Union Carbide commissioned the first prototype gas-phase HDPE plant in Seadrift, Texas in 1968. Early commercial breakthroughs included the first third-party license issued to Chemopetrol in 1972 and the world’s first LLDPE UNIPOL process plant in 1977.
Key technological milestones include the commercialization of condensed mode technology by Union Carbide in 1984, which introduced Induced Condensing Agents (ICAs) to enhance heat removal capacity. ExxonMobil subsequently developed Super Condensed Mode Technology (SCM-T) in the 1990s, enabling ICA concentrations up to 50% mass fraction and production capacity increases exceeding 2.5× baseline.
The formation of Univation Technologies in 1997 as a joint venture between Union Carbide and ExxonMobil combined UNIPOL process technology with EXXPOL metallocene catalysts and SCM-T capabilities. Dow Chemical acquired complete ownership in 2015. As of mid 2025, the technology operates in over 190 reactor lines across 25+ countries, producing approximately one-third of global HDPE and LLDPE.
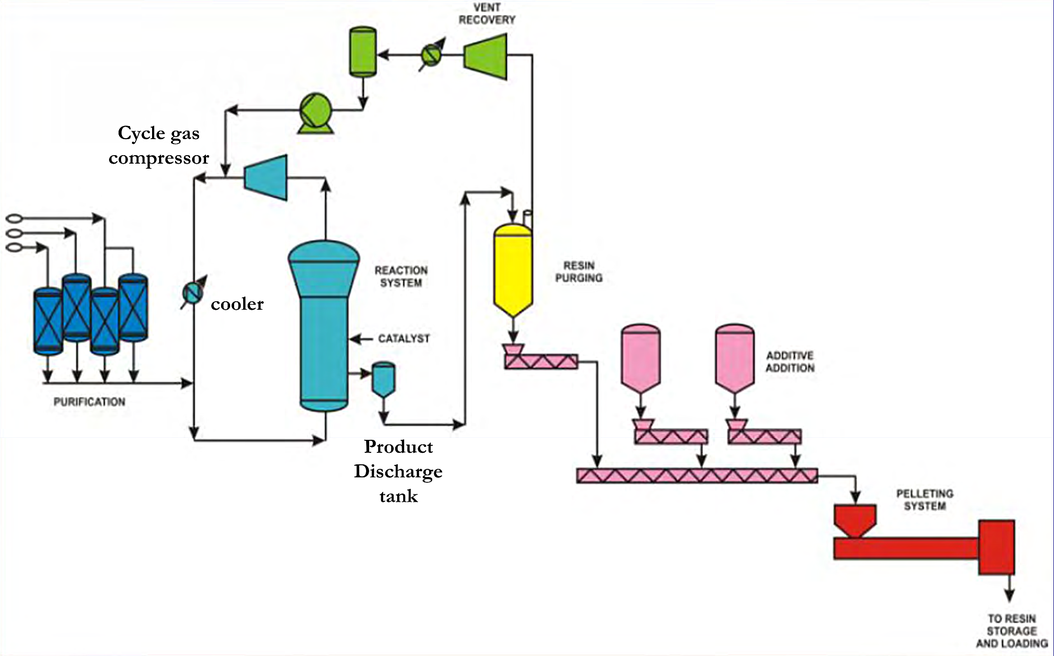
Figure 1 - History of Unipol PE technology, 1968-2016
Technology Summary
The UNIPOL™ PE Process is a gas-phase fluidized bed polymerization system for producing polyethylene grades with densities from 0.900-0.970 g/cm³ and melt indices from 0.01-150 g/10 min. The process supports multiple catalyst technologies including Ziegler-Natta, chromium, metallocene and post-metallocene systems.
The technology operates in three modes: dry (baseline capacity), condensed (1.6× capacity), and super-condensed (2.5×+ capacity). Recent designs achieve single reactor capacities up to 800,000 tonnes/year. The process accommodates both C4 (1-butene) and C6 (1-hexene) comonomers, with catalyst-comonomer selection affecting both product properties and reactor throughput.
Detailed Technology Description
Process Configuration
The UNIPOL™ process consists of the following main equipment:
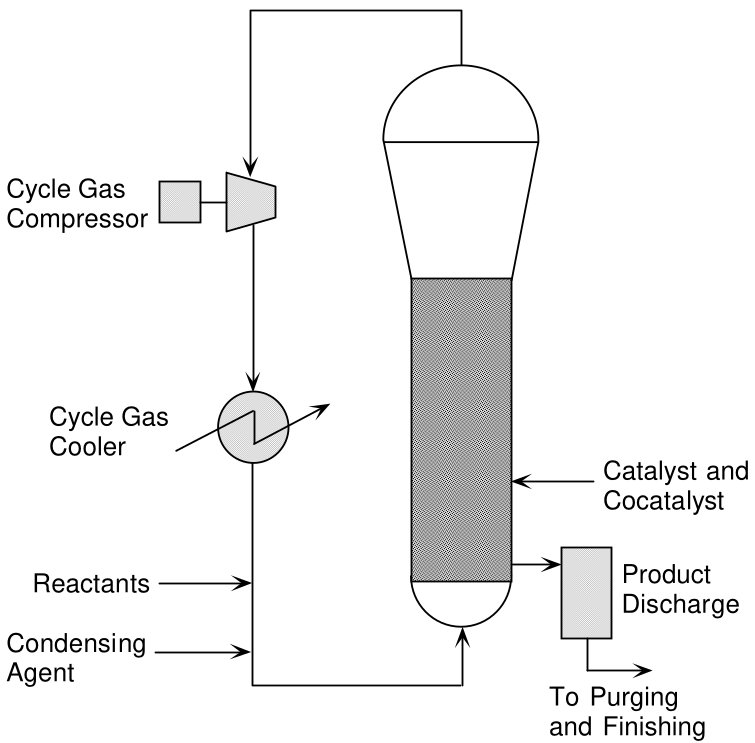
- Fluidized bed reactor: Vertical cylindrical vessel operating at 70-100°C and 1-2 MPa
- Cycle gas compressor: Circulates reactant gases through the polymer bed
- Heat exchangers: Remove polymerization heat from the cycle gas stream
- Product degassing system: Removes entrained hydrocarbons from polymer particles
- Pelletizing system: Converts polymer particles to commercial pellets
Operating Modes
- Dry Mode: Heat removal via sensible heat of cycle gas only. Provides baseline reactor capacity with maximum operational stability. Used during startup operations, during grade transitions, for process trouble shooting, and with certain catalyst systems performing better without ICAs.
- Condensed Mode: Introduces Induced Condensing Agents (ICAs) such as isopentane, hexane, or propane into the cycle gas. These compounds partially condense in heat exchangers and evaporate in the reactor, utilizing latent heat of vaporization for enhanced heat removal. Typical condensate fraction: 15-25% of cycle gas mass. Capacity increase: up to 60%. Widely practiced due to broad catalyst compatibility combined with well-understood operation control.
- Super Condensed Mode (SCM-T): Employs ICA concentrations up to 50% mass fraction. Creates cosolubility effects that enhance ethylene solubility in the polymer phase, improving both heat removal and polymerization kinetics. Capacity increase: 2.5× or greater. Particularly effective with metallocene catalysts. More complex control and monitoring needs must be offset by market premiums.
Figure 2 - Process Schematic of a Unipol™ Gas Phase Reactor System showing the injection of an Induced Condensing Agent (ICA)
Modern UNIPOL PE plants operate as flexible, multi-mode systems rather than exclusively in super-condensed mode. The selection of operating mode depends on a complex optimization involving product requirements, catalyst selection, market conditions, and operational considerations. This flexibility is touted by the technology licensor as a key competitive advantage of UNIPOL technology, allowing producers to optimize their operations dynamically rather than being locked into a single operational approach.
Process Parameters
- Temperature: 70–100°C (Depending on PE grade and catalyst)
- Pressure: ~1–2 MPa (Optimized for catalyst and comonomer incorporation)
- Condensing Agent: Isopentane, hexane (fraction adjustable to maximize capacity)
- Reaction time: 1-5 hours
- Ethylene conversion per pass: ~2-5%
- Polymer particle size: 500-1500 microns
Catalyst Systems
- Conventional Catalysts: Ziegler-Natta and chromium-based systems produce broad molecular weight distribution polymers suitable for standard applications.
- Metallocene Catalysts: Single-site catalysts producing narrow molecular weight distribution polymers with enhanced properties.
Comonomer Selection
- C4 (1-Butene): Standard comonomer for conventional catalysts. Provides optimal reactor productivity and economics.
- C6 (1-Hexene): Preferred for metallocene catalysts despite throughput penalties. Enables superior impact strength, optical properties, and processing characteristics.
Process Efficiency and Yields: Standard, Condensed, Super-Condensed Modes
Comparative process efficiency of the UNIPOL™ PE Process, as claimed by the technology licensor, include capital costs 15-25% lower than slurry loop systems, achieved through simplified equipment requirements with only three major rotating pieces. Operating costs are also claimed to be up to 10% lower than alternatives due to elimination of solvent handling systems and direct product recovery without intermediate storage.
Commercial Experience
Over 190 licensed reactor lines operate worldwide, producing approximately one-third of global HDPE and LLDPE capacity according to the technology licensor. Recent capacity achievements include single reactors designed for 800,000 tonnes/year.
References
- Univation Technologies. Unipol Process. (accessed Aug 27, 2025)
- Univation Technologies. History of Unipol™ PE Process. (accessed Aug 27, 2025)
- Univation Technologies. About: History. (Retrieved via The Web Archive, Captured on Nov 20, 2018)
- Univation Technologies. Unipol: Overview. (Retrieved via The Web Archive, Captured on Sep 18, 2018)
- Univation Technologies. May 12, 2025. Univation Technologies Announces World-Scale 800,000 Tonnes/Year Design Capacity for UNIPOL™ PE Process Technology.
- Echem Enppi. Apr 2013. Polyethylene Production Technologies. (Retrieved via The Web Archive, Captured on Nov 12, 2019)
- Achmad Amiruddin Hasan. Document dated: Jul 3, 2015. Preliminary Design of Linear Low Density Polyethylene (LLDPE) Using UNIPOL Process Capacity of 400,000 Tons/Year. Universitas Muhammadiyah Surakarta. Institutional Repository. Chapter 1, Introduction.
- European Commission. Aug 2007. Reference Document on Best Available Techniques in the Production of Polymers.
- W.A. Fraser et al.. Oct 1997. Technology Bulletin: Manufacturing Efficiencies From Metallocene Catalysis In Gas-Phase Polyethylene Production. Univation Technologies. (Retrieved via SCRIBD)
- Timothy McKenna, Arash Alizadeh. May 2015. The impact of induced condensing agents in the gas phase polymerization of ethylene. ECI Digital Archives. Polymer Reaction Engineering IX (2015).
- Timothy F.L. McKenna et al.. Document date: Feb 14, 2019. Improved Understanding of the Impact of Alkaneswhen uisng Condensed Mode Cooling for PE Production. SPE ANTEC 2019. (Retrieved via Events Cloud)
- Ian D. Burdett, Ronald S. Eisinger. Oct 10, 2017. Ethylene Polymerization Processes and Manufacture of Polyethylene. Handbook of Industrial Polyethylene and Technology. ISBN: 9781119159797. DOI: 10.1002/9781119159797.ch3.
-
-
-
-