Introduction
Paraffins isomerization represents a critical process in petroleum refineries and petrochemical complexes for upgrading light hydrocarbon streams and meeting stringent fuel quality requirements. The process converts straight-chain paraffin molecules into their branched isomers through skeletal rearrangement, maintaining the same molecular formula but achieving improved fuel properties and higher value products. Catalytic isomerization processes that use hydrogen have been developed to operate under moderate conditions. This versatility has made isomerization increasingly important as environmental regulations limit aromatic content and eliminate lead additives from gasoline.
Figure 1 - Block Flow Diagram of a typical Isomerization Unit[3]
Feedstock Diversity and Processing Applications
C5-C6 Light Naphtha Isomerization
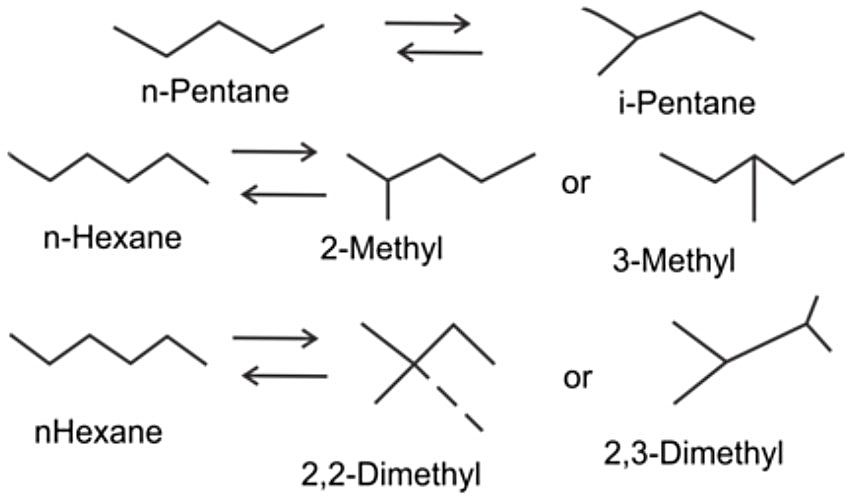
Typical feedstocks for isomerization process include hydrotreated light straight-Run Naphtha, light natural Gasoline, or condensate. The process addresses octane enhancement of light gasoline fractions, converting normal paraffins with research octane numbers (RON) of approximately 62 (n-pentane) and 25 (n-hexane) to their branched isomers with significantly higher octane ratings of 92 and 100 respectively. This process serves as a critical complement to catalytic reforming by upgrading light fractions that would otherwise undergo undesirable hydrocracking or benzene formation in reformers.
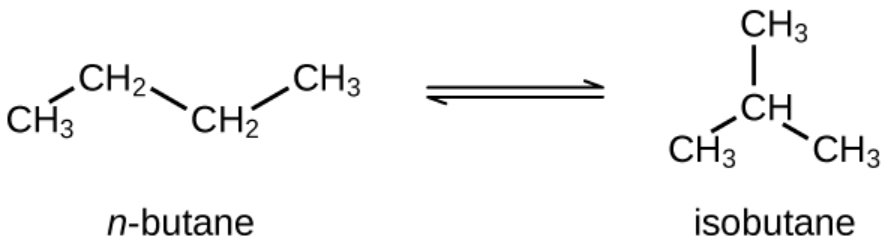
n-Butane (C4) Isomerization
Normal Butane most commonly originates from the liquefied–petroleum-gas (LPG) streams recovered in refineries and natural-gas-liquids (NGL) plants. Typical sources include the de-ethaniser/de-propaniser section of an NGL fractionation train, where C4-rich bottoms are split into n-/iso-butane streams, and refinery stabiliser or debutaniser columns that remove light ends (C3/C4) from reformate, FCC gasoline and hydrotreating units. Isomerization if n-butane into isobutane represents a fundamental process for producing high-purity iso-paraffins serving as a feedstock for alkylation units, which generate high-octane gasoline blending components. Isobutane can also be dehydrogenated into isobutylene used as a feedstock for the production of methyl tert-butyl ether (MTBE), ethyl tert-butyl ether (ETBE), and butyl rubber.
C7 Fraction Processing
C7 hydrocarbon fractions, representing 10-15% of wide gasoline fractions, present unique opportunities for isomerization processing. These components typically exhibit poor performance in reforming units due to unfavorable dehydrocyclization kinetics compared to hydrocracking rates. Advanced isomerization technologies can convert C7 paraffins with RON of 55-65 into non-aromatic gasoline components achieving octane numbers of 83-85.
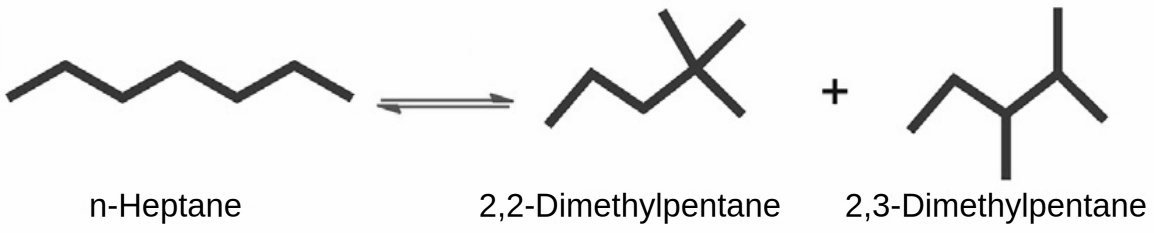
Reverse Isomerization and Normalization
An emerging application involves "reverse isomerization" or normalization of C4 and C5 paraffin hydrocarbons to produce linear paraffins suitable for dehydrogenation units producing olefins suitable as comonomers in subsequent polymer synthesis. This process direction demonstrates the flexibility of modern isomerization technology in meeting diverse petrochemical requirements.
Catalyst Systems and Technologies
Paraffin isomerization is fundamentally an equilibrium-limited reaction where straight-chain paraffins are converted to their branched isomers through skeletal rearrangement. The thermodynamic equilibrium strongly favors branched isomers at lower temperatures, making catalyst activity crucial for achieving both acceptable reaction rates and maximum conversion to high-octane products.
Chlorinated Alumina Catalysts
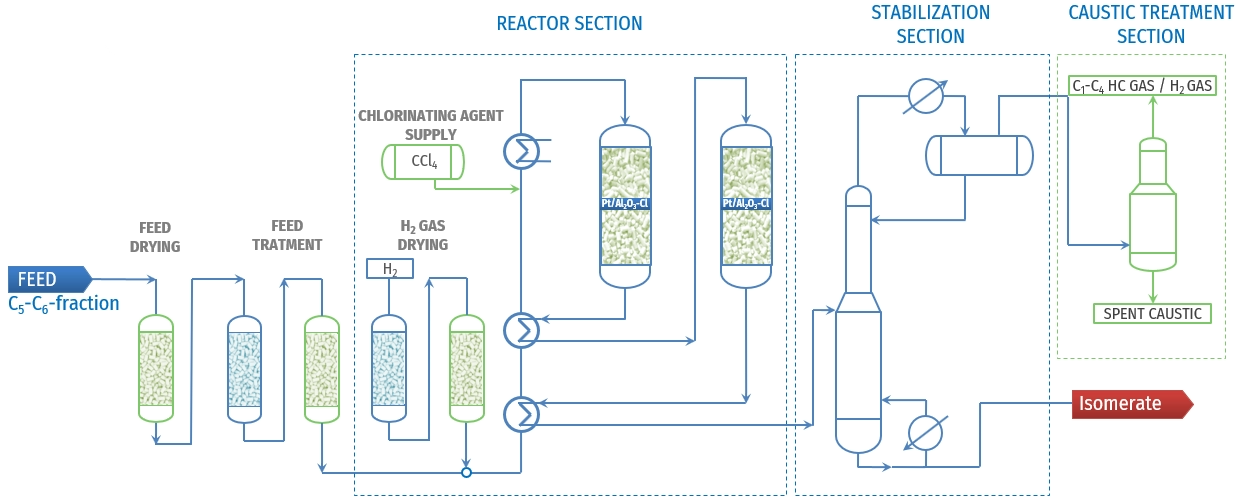
Platinum-supported chlorinated alumina represents the most active catalyst system for paraffin isomerization, enabling operation at relatively low temperatures (120-175°C) that favor thermodynamic equilibrium toward branched isomers. These catalysts achieve high selectivity and conversion but require continuous chlorinating agent supply, creating environmental concerns and operational complexity including caustic scrubbing systems and waste handling.
The high activity stems from strong acid sites generated by chlorine promotion, but this also creates extreme sensitivity to water, sulfur, and nitrogen compounds. Catalyst replacement typically occurs every 3-5 years, with specialized reactor designs featuring dual parallel reactors to enable catalyst changeout without process shutdown.
Sulfated Metal Oxide Catalysts
Sulfated zirconia and related metal oxide catalysts emerged as environmentally friendly alternatives, offering moderate to high activity without chlorinating agent requirements. These catalysts operate at slightly higher temperatures (130-220°C) but provide regenerability and improved tolerance to feed contaminants. Platinum-promoted sulfated zirconia systems demonstrate particular promise, combining reasonable activity with operational robustness.
Recent developments include advanced sulfated zirconia formulations achieving activity levels approaching chlorinated systems while maintaining environmental advantages. These catalysts enable simplified process designs without caustic treatment systems and reduced chemical consumption.
Zeolite-Based Catalysts
Zeolitic isomerization catalysts operate at higher temperatures (250-280°C) with inherently lower activity due to thermodynamically unfavorable conditions, but offer complete regenerability and insensitivity to water and oxygenates. While less commonly selected for new installations due to octane limitations, zeolite catalysts find application in unit conversions and specialized processing scenarios.
Dual-Function Catalyst Design
Modern catalyst systems employ dual-function designs incorporating platinum for hydrogenation/dehydrogenation reactions and acid sites for isomerization. This bifunctional approach optimizes reaction pathways while minimizing side reactions such as cracking and coke formation. Advanced formulations continue evolving to enhance metal-acid balance and improve catalyst longevity.
Process Configurations and Integration
Universal Process Flow Configurations
Once-Through Operations provide the most economical approach with minimal capital investment, suitable for moderate octane enhancement across all paraffin ranges. Fresh feed combined with make-up hydrogen is heated to reaction temperature and directed to fixed-bed reactors containing bifunctional catalysts. This configuration achieves:
- C4 isomerization: 50-55% single-pass conversion at 160-210°C and 1.5-2.0 MPa
- C5-C6 processing: RON improvement from 70 to 82-84 at 120-175°C and 2.8-3.8 MPa
- C7 upgrading: RON enhancement from 55-65 to 83-85 at similar conditions
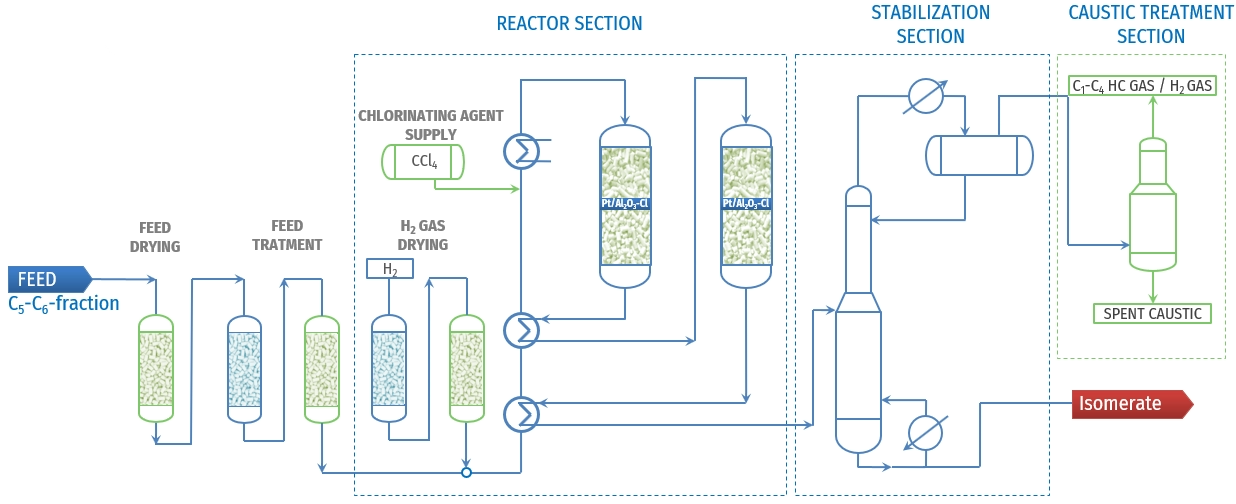
Figure 2 - Typical PFD of “once-through” isomerisation unit using chlorinated catalyst[4]
Enhanced Recycle Configurations incorporate separation and recycle of unconverted normal paraffins to maximize conversion and product quality. These systems employ various separation technologies:
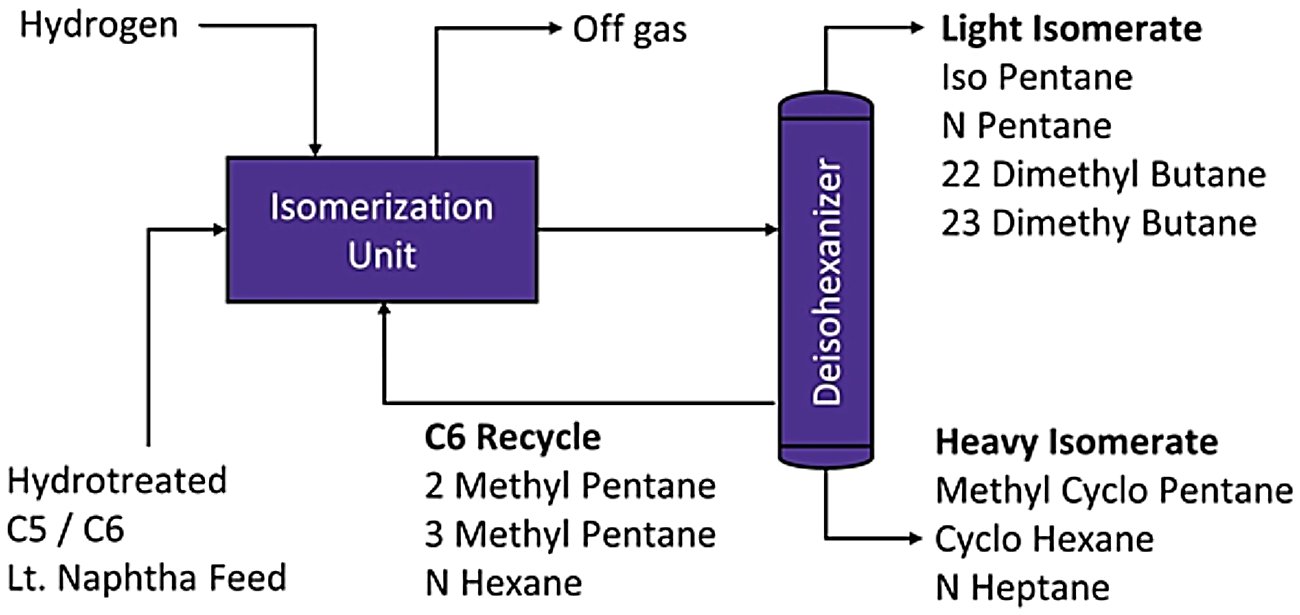
- Fractionation schemes: Deisopentanizer (DIP), deisohexanizer (DIH), and depentanizer (DP) columns enable selective recycle of specific paraffin cuts
- Adsorptive separation: Molecular sieve systems (such as vapor-phase adsorption processes) selectively remove normal paraffins for recycle
- Integrated separation: Super-DIH configurations combine multiple separation duties in single columns for capital efficiency
Figure 3 - Typical Configuration for producing Hexane Products in Industry using conventional sequence of columns[3]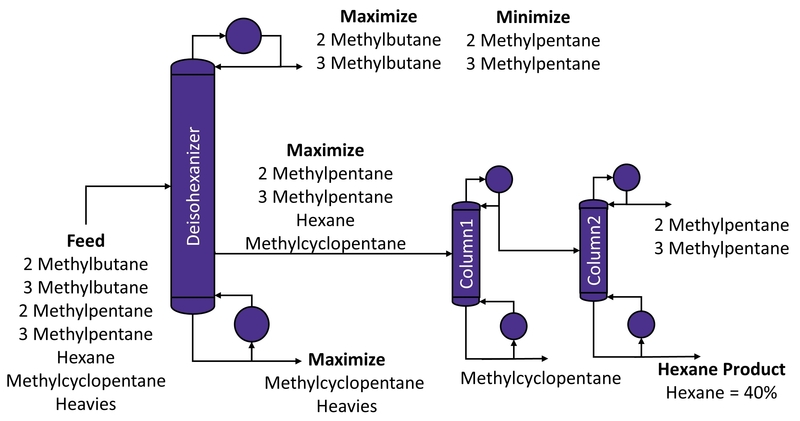
Operating Parameters by Feedstock Type
C4 Butane Isomerization:
- Temperature: 160-210°C (higher than C5/C6 due to thermodynamic requirements)
- Pressure: 1.5-2.0 MPa (lower than other systems due to gaseous nature)
- Single-pass conversion: 50-55%
- H2/HC molar ratio: 0.07-0.15
Figure 4 - Isomalk-3 n-Butane Isomerization Process Flow Diagram[6]
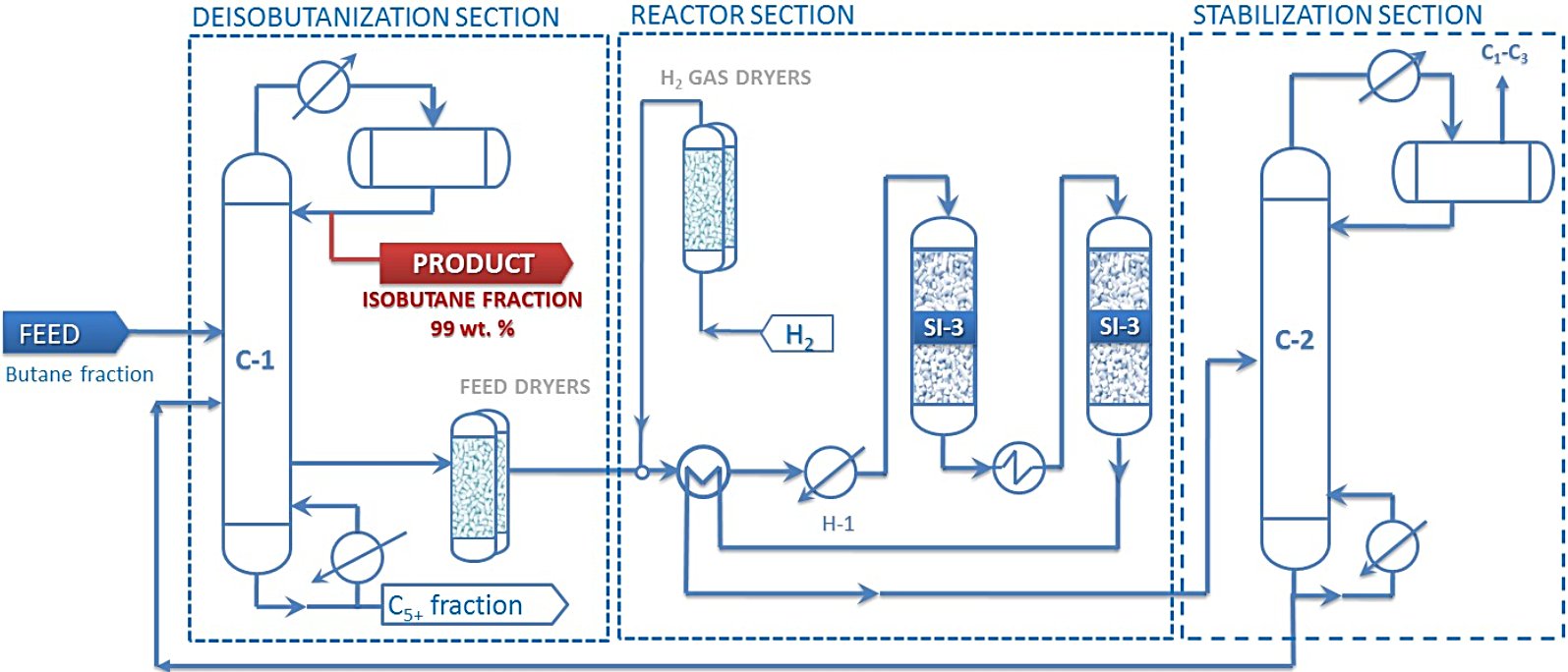
Legend: Isomerization reaction proceeds over SI-3 catalyst in the presence of hydrogen. Isomalk-3 process includes the following stages: 1. Separation into iso- and n-butane fractions, separation of C5+ hydrocarbons in deisobutanizer section. 2. Feed and hydrogen treatment in dryers to remove undesirable impurities. 3. Butane fraction isomerization reactions over SI-3 catalyst in one or several reactors in series. 4. Separation of dissolved hydrogen and light hydrocarbons (C1-C3) from product in stabilization column section (C-2) and sending stabilized product to fractionation column section (C-1) to produce high-purity isobutane. Unconverted n-butane is recycled back to isomerization reactor section, thereby ensuring maximum conversion of n-butane to isobutane.
C5-C6 Light Naphtha Processing:
- Temperature: 120-175°C (thermodynamically favorable range)
- Pressure: 2.8-3.8 MPa
- Once-through RON: 82-84; Recycle RON: 87-93
- Typical yields: >97 wt% C5+ product
- LHSV: 1.0-3.0 h-1 depending on catalyst system
C7 Heavy Paraffin Isomerization:
- Temperature: Similar to C5-C6 range (120-175°C)
- Pressure: 2.8-3.8 MPa
- RON improvement: 55-65 → 83-85
- Yields: Typically 95-98% C7+ retention
Reverse Isomerization/Normalization:
- Objective: Convert branched C4-C5 paraffins to linear forms
- Operating conditions: Modified to favor normal paraffin equilibrium
- Temperature optimization: Higher temperatures may favor normal paraffin formation
Advanced Process Integration
Modern isomerization units often integrate multiple feedstock processing capabilities within single facilities, enabling refiners to optimize product slate based on market conditions. Flexible reactor systems can process different paraffin cuts through campaign operations or parallel processing trains.
Multi-reactor configurations enable temperature profiling to optimize thermodynamic driving force while maintaining reaction rates. The first reactor operates at higher temperature for kinetic advantage, while subsequent reactors operate at lower temperatures for equilibrium optimization.
Heat integration recovers reaction heat for feed preheating, improving overall process efficiency. Since isomerization reactions are mildly exothermic, careful temperature control prevents excessive temperature rise that would unfavorably shift equilibrium.
Product Optimization Strategies
The equilibrium nature of isomerization enables product optimization through:
- Separation severity: More aggressive separation of normal paraffins increases recycle ratio and ultimate conversion
- Operating temperature profiles: Multi-reactor systems optimize kinetic and thermodynamic trade-offs
- Catalyst selection: Higher activity catalysts enable lower temperature operation, improving equilibrium conversion
- Pressure optimization: While conversion is pressure-independent, pressure affects catalyst life and separation efficiency
Economic and Market Drivers
Isomerization economics depend heavily on octane value differentials, product specifications, and feedstock availability. The process provides particular value in regions with stringent gasoline specifications limiting aromatic content while maintaining octane requirements. Market dynamics including shale gas development and changing refinery configurations continue influencing isomerization technology selection and deployment.
Commercial applications demonstrate consistent profitability with typical processing fees of 10-12 cents per gallon for merchant operations, while integrated refinery applications capture full octane value premiums. Technology selection requires careful consideration of feedstock characteristics, product specifications, environmental constraints, and economic optimization.
Conclusion
Paraffins isomerization encompasses diverse applications extending well beyond traditional high-octane isomerate production. The technology addresses multiple feedstock types (C4, C5-C6, C7+ paraffins) through various processing directions including both conventional isomerization and reverse normalization processes. Catalyst systems continue evolving from chlorinated alumina toward more environmentally sustainable sulfated oxide and zeolite formulations, while process configurations adapt to changing market requirements and environmental regulations.
The strategic importance of isomerization in modern refining reflects its role in producing clean, high-quality fuels while supporting petrochemical feedstock requirements. Continued technological advancement and economic optimization ensure paraffins isomerization remains a cornerstone process for sustainable hydrocarbon utilization.
References
- Naqvi, S.R., et al. "New trends in improving gasoline quality and octane through naphtha isomerization: a short review." Applied Petrochemical Research, vol. 8, 2018, pp. 131-139.
- Sullivan, D., Metro, S., and Pujadó, P.R. "Isomerization in Petroleum Processing." Handbook of Petroleum Processing, Springer International Publishing Switzerland, 2015, pp. 479-497.
- DWC Innovation. Whitepaper : Maximize Margins in Light Naphtha Isomerization Unit using Dividing Wall Column Technology.
- Shakun, A.N., et al. "Development of paraffin hydrocarbons isomerisation processes." Digital Refining, August 2021.
- Buffin, G., et al. "Isomerisation: opportunities for a more sustainable refining industry." Hydrocarbon Engineering, November 2023.
- SIE Neftehim. "N-butane Isomerization ISOMALK-3." Available: https://nefthim.com/developments/butane-isomerization-isomalk-3/
- Springer Link. "New trends in improving gasoline quality and octane through naphtha isomerization." Available: https://link.springer.com/article/10.1007/s13203-018-0204-y
- EgyOil-Gas. "The Science of Isomerization: Enhancing Gasoline Production at Midor." Available: https://egyptoil-gas.com/features/the-science-of-isomerization-enhancing-gasoline-production-at-midor/
- Chandra Asri. "Refining Crude Oil Process, from Separation to Treatment." Available: https://chandra-asri.com/en/blog/refining-crude-oil
- IJRES. "Isomerization process carried out in Petroleum Refining." International Journal of Research in Engineering and Science, vol. 10, no. 5, 2022.
- Axens. "C5/C6 Isomerization." Available: https://www.axens.net/expertise/oil-refining/c5c6-isomerization
- Penn State University. "Isomerization." FSC 432: Petroleum Refining. Available: https://www.e-education.psu.edu/fsc432/content/isomerization
- European Patent Office. "Methods and systems for paraffin isomerization and separation." EP4101910A1, 2022.
- Set Laboratories. "Isomerization." Available: http://www.setlab.com/resources/refining/isomerization/
- Weitkamp, J., and Puppe, L. "Catalysis and Zeolites - Fundamentals and Applications." Springer, 1999.
- Chekantsev, N.V., et al. "Mathematical modeling of light naphtha (C5, C6) isomerization process." Chemical Engineering Journal, vol. 238, 2014.
- RBN Energy. "You Can Just Iso my Butane: Isobutane and Isomerization." 2022.
- SIE Neftehim. "C7-fraction Isomerization ISOMALK-4." Available: https://nefthim.com/developments/Light-gasoline-fractions-isomerization/
- Artina Azma Mehr. "Isomerization process catalyst." Available: https://artinazma.net/en/isomerization-process-catalyst/
- Giliard, N. "Preparation and characterization of sulfated zirconia catalysts." Max Planck Institute Dissertation.
- Google Patents. "Chlorinated, alumina-based, bimetallic catalyst." US5446230A, 1995.
- Yang, S., et al. "Role of Sulfation of Zirconia Catalysts in Vapor Phase Ketonization." Journal of Physical Chemistry C, vol. 125, 2021.
- IJARSCT. "Enhancement of Octane Number of Gasoline by Isomerization Process." Technical Review.
- Kumar, A., et al. "Synthesis of sulfated zirconia-HY zeolite catalysts doped with platinum." Journal of Ecological Engineering, vol. 26, no. 3, 2025.
- Pope, T.D., et al. "A study of catalyst formulations for isomerization of C7 hydrocarbons." Applied Catalysis A: General, vol. 233, 2002, pp. 45-62.
- Wang, J., et al. "Bottom-Up Synthesis of Platinum Dual-Atom Catalysts on Cerium Oxide." ACS Catalysis, vol. 14, 2024.
- He, S., et al. "Optimizing the dehydrogenation catalyst of higher normal paraffins." Catalysis Science & Technology, vol. 6, 2016.
- Google Patents. "Dual function catalysts." US2886536A, 1959.
- He, S., et al. "Kinetics of long chain n-paraffin dehydrogenation over a commercial Pt-Sn-K-Mg/γ-Al2O3 catalyst." Applied Catalysis A: General, vol. 579, 2019.