Historical Development
Adiponitrile’s first industrial route (1950s–1960s) relied on multi-step chlorocyanation of butadiene; yielding admixtures of dinitriles, low selectivity, and substantial wastes. In 1967, William C. Drinkard at DuPont introduced direct butadiene hydrocyanation using zero-valent nickel–phosphite catalysts, rapidly earning predominance due to simplified operations and superior atom economy[1][2].
Process Chemistry
The net reaction converts 1,3-butadiene and two equivalents of hydrogen cyanide (HCN) into adiponitrile:
H2C=CH−CH=CH2 + 2 HCN → NC–(CH2)4–CN
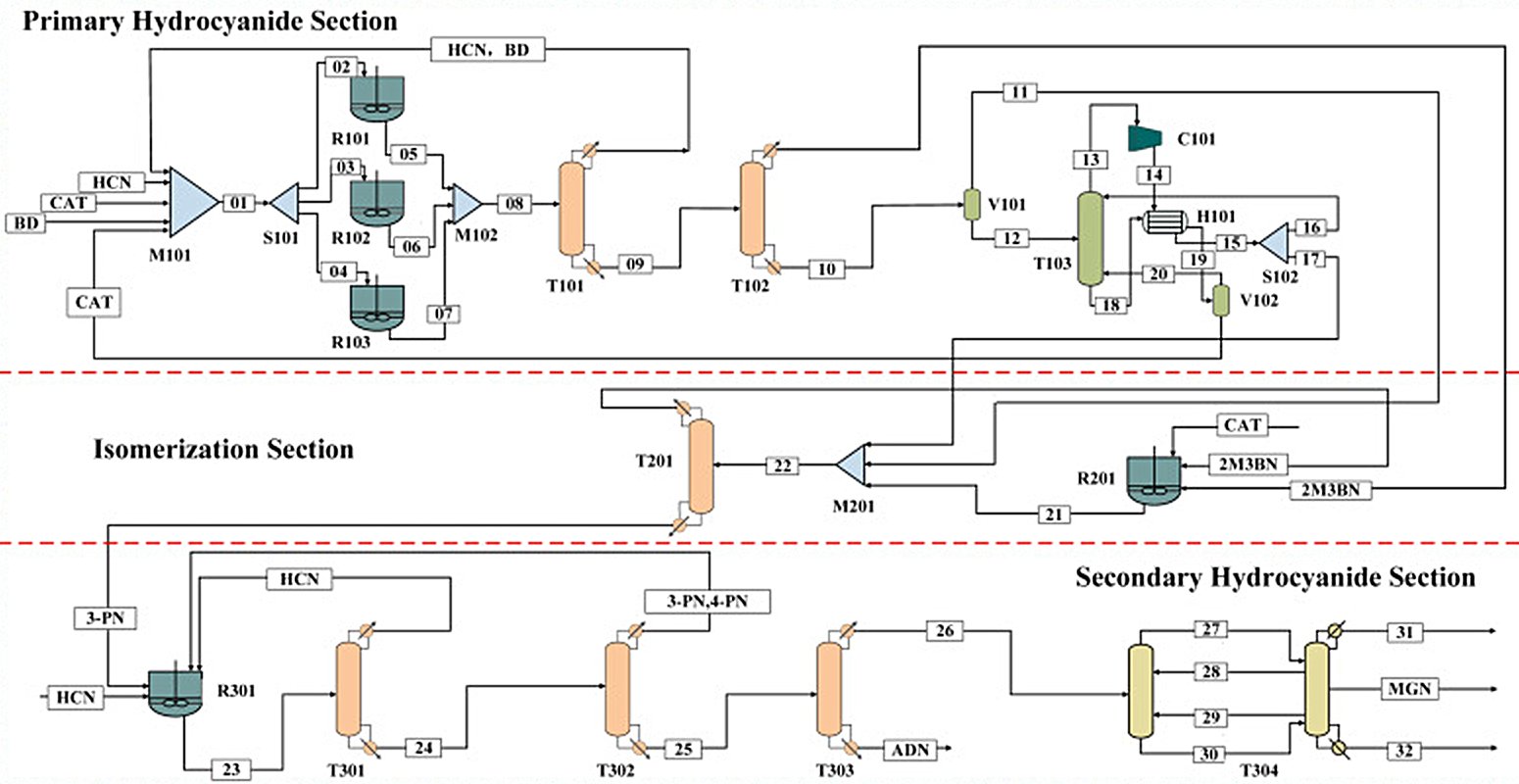
The process reactions involve (Fig. 1):
| |
The formation of 3-pentenenitrile (3PN) and 2-methyl-3-butenenitrile (2M3BN) (approx. 2:1 ratio) via π-allyl intermediates during the primary hydrocyanation[1][3][4][5]
|
(a) |
| |
The rearrangement of branched 2M3BN to linear 3PN via reversible C–CN activation during isomerization step (dehydrocyanation/recyanation cycles are confirmed by deuterium-labelling studies)[1][6][7]
|
(b) |
| |
The adddition of second HCN across the C=C bond of 3PN to yield adiponitrile (ADN) and minor 2-methylglutaronitrile[1][3][4][5] |
(c) |
Figure 1 - Formation of 3-pentenenitrile (3PN) and 2-methyl-3-butenenitrile (2M3BN) (approx. 2:1 ratio) via π-allyl intermediates[1]
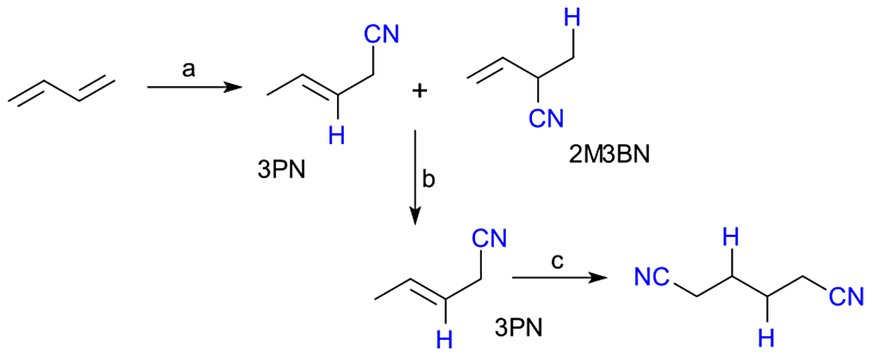
Academic and industrial mechanistic studies in the 1970s–1990s elucidated the catalytic cycle (Fig. 2)—oxidative addition of HCN to Ni(0), migratory insertion of butadiene to form π-allyl–nickel cyanide, and reductive elimination of pentenenitriles—guiding ligand innovations and process intensification[1][3][4][5].
Figure 2 - Proposed cycle for the concurrent butadiene hydrocyanation and 2-methyl-3-butenenitrile (2M3BN) isomerization[3]
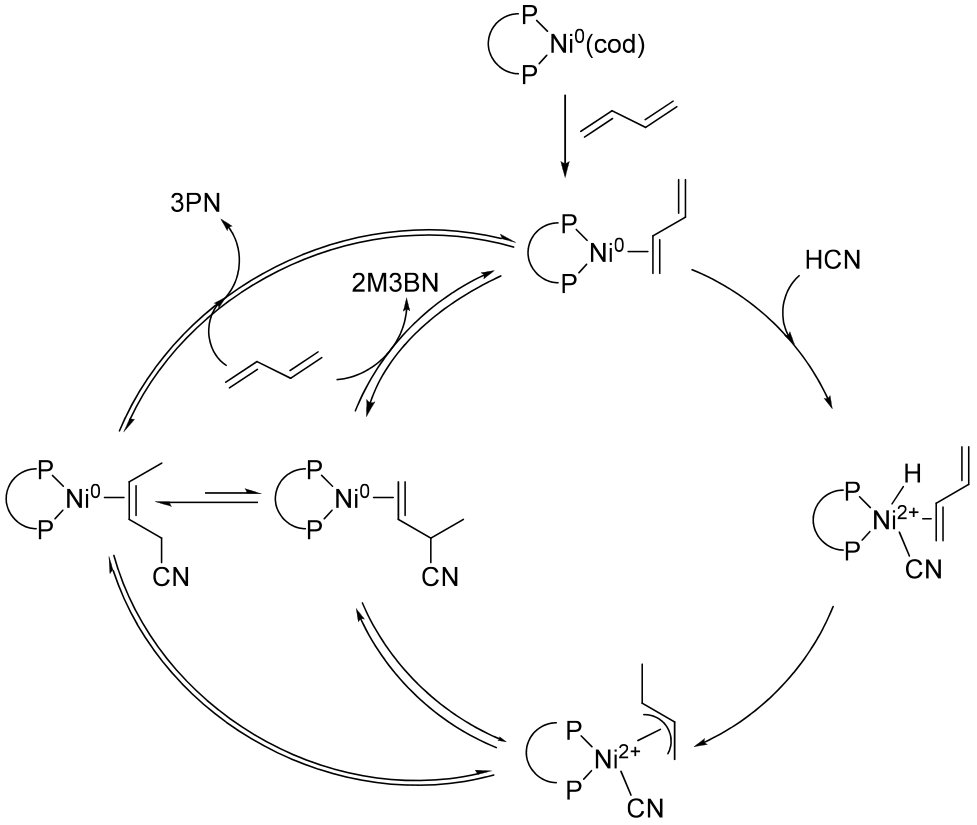
Detailed Step-by-Step Process Description
A typical industrial scheme comprises three reactors in series, followed by catalyst recovery and distillation (block flow diagram illustration).
- Primary Hydrocyanation
- Reactants: Butadiene, HCN, Ni–phosphite catalyst (e.g., Ni[P(OC6H5Me-p)3]4)
- Conditions: 80–130 °C, 5–20 bar[9][10]
- Isomerization
- Catalysts/Co-catalysts: Same Ni complex with ZnCl2 or alternative Lewis acid
- Conditions: 60–120 °C, 1–10 bar
- Secondary Hydrocyanation
- Reactants: Recycled 3PN, HCN, Ni catalyst, Lewis acid (commonly AlCl3)
- Conditions: 30–130 °C, 1–20 bar
- Product Purification: Multistage distillation achieves >99.5% ADN purity[11][12][13]
Table 1 - Typical Conditions and Outputs
| Step |
Temp (°C) |
Pressure (bar) |
Catalyst
System |
Key
Intermediates/
Products |
| Primary Hydrocyanation |
80–130 |
5–20 |
Ni[P(OC6H5Me-p)3]4 |
3PN (∼65%), 2M3BN (∼35%) |
| Isomerization |
60–120 |
1–10 |
Ni[P(OC6H5Me-p)3]4 + ZnCl2 |
3PN (via 2M3BN
→ 3PN) |
| Secondary Hydrocyanation |
30–130 |
1–20 |
Ni[P(OC6H5Me-p)3]4 + AlCl3 |
Adiponitrile (97–99% yield) |
Process Efficiency
- Overall Yield: 97–99% of theoretical[1][5]
- Single-Pass Selectivity: 81–87% to adiponitrile, with by-product minimization via recycling of intermediates[5]
- Product Purity: >99.5% after distillation
Lewis acid choice significantly influences relative rates (k3/k4) and steady-state intermediate distributions (Table 2):
Table 2 - Lewis Acid Effects on Reaction Rates at 68 °C[5]
| Lewis Acid |
[4PN]/[3PN]
SS (%) |
Relative HL
Rates (k3:k4) |
Adiponitrile
Selectivity (%) |
| AlCl3 |
0.5 : 99.5 |
365 : 678 |
47.7 |
| ZnCl2 |
3.0 : 97.0 |
220 : 1470 |
81.8 |
| BPh3 |
7.0 : 93.0 |
39 : 1260 |
90.5 |
Economic Performance
- Capital Expenditure: A world-scale (∼200 kt/y) plant requires 400–600 M USD for in-side battery limits (ISBL), with off-site facilities and start-up adding 30–50%
- Operating Costs Breakdown:
- Butadiene (40–45%)
- HCN (15–20%)
- Catalyst & Ligand makeup (5–10%)
- Utilities & overhead (25–30%)
- Minimum Sustainable Selling Price: Typically 1,700–1,900 USD/t, competitive with alternative ADN routes[14]
Process intensification studies (e.g., hybrid modeling, ML-aided simulation) suggest 10–15% reductions in energy and raw-material consumption possible without major capital additions[8].
Proprietary Technologies and Licensors
Table 2 - Major technology owners and licensors
| Technology/Process |
Licensor |
Notes |
| INVISTA Process |
INVISTA |
Original developer;
monodendate phosphite catalyst |
| Butachimie (JV) |
BASF &
INVISTA |
French JV; capacity ∼600 kt/y
after 2022 expansion |
| BASF SE Process |
BASF |
Proprietary ligand optimizations; Chalampé plant |
| Asahi Kasei Process |
Asahi Kasei |
Japanese variant;
minor EHD* integration |
| Kishida Chemical Process |
Kishida Chemical, Japan |
Small-scale EHD* route |
*Electrohydrodimerization (EHD) of Acrylonitrile
Catalyst formulations (e.g., diphosphite, diphosphonite ligands) are heavily patented and trade-secret-protected, enabling incremental performance gains in selectivity and lifetime[9][10][11].
The Direct Butadiene Hydrocyanation controls >90% of global ADN capacity, while Electrohydrodimerization (EHD) of Acrylonitrile has ∼5% market share (notably Asahi Kasei, Kishida Chemical). Adipic Acid Ammoniation/Dehydration is <5% and regionally deployed (e.g., BASF gas-phase units). Other Routes such as chlorocyanation or caprolactam degradation are marginal and largely obsolete[6][7][8].
References
- Hydrocyanation - Wikipedia
- Adiponitrile - Wikipedia
- Laura Bini. (2009). Mechanistic insights into the hydrocyanation reaction. [Phd Thesis 1 (Research TU/e / Graduation TU/e), Chemical Engineering and Chemistry]. Technische Universiteit Eindhoven. DOI: 10.6100/IR644067.
- Wenwen Cong et al., Process intensification and economic evaluation of adiponitrile production based on hybrid modeling, Chemical Engineering and Processing - Process Intensification, Vol. 213, (2025), 110325, ISSN 0255-2701, DOI: 10.1016/j.cep.2025.110325.
- Zhang S. et al. (2019). Method for preparing adiponitrile using dialkyl adipate and ammonia via catalytic ammonolysis-dehydration. Chinese Patent CN 110511162 A, published Nov 29, 2019, application filed Jun 11, 2019, Institute of Process Engineering, Chinese Academy of Sciences.
- D.E. Blanco et al. (2019). Enhancing selectivity and efficiency in the electrochemical synthesis of adiponitrile. Reaction Chemistry and Engineering, 4(1), 8-16. DOI: 10.1039/c8re00262b.
- Hydrocyanation - CHEMEUROPE.COM
- Adiponitrile Market - Forecast(2025 - 2031) - IndustryARC
- Piet van Leeuwen et al. (2003). Process for the hydrocyanation of butadiene. European Patent EP1344770A1, published Sep 17, 2003, application filed Mar 10, 2003, Invista Technologies Saerl.
- Laura Bini et al. Ligand development in the Ni-catalyzed hydrocyanation of alkenes. Chemical Communications, vol. 46, no. 44, 2010, pp. 8325–8334. The Royal Society of Chemistry. DOI: 10.1039/C0CC01452D.
- J.M. Gartner et al. Hydrocyanation of 2-pentenenitrile. U.S. Patent US20120035387A1, filed Mar. 14, 2011; published Feb. 9, 2012. Invista North America LLC - Inv Nylon Chemicals Americas LLC.
- S. Zhang, et al. [Method for preparing adiponitrile by direct hydrocyanation of butadiene]. Chinese Patent CN 111892514 A, filed Aug 13, 2020; published Nov 6, 2020. Yangquan Coal Group Design And Research Center Co ltd - Yangquan Coal Industry Group Co Ltd
- Alexander P. V. Göthlich et al. Syntheses, Structures, and Nickel-Catalyzed Hydrocyanation of Conjugated Dienes. Organometallics 2007, 26 (5), 1184–1186. DOI: 10.1021/om701140c.
- Q3 2024 - Adiponitrile Production from Butadiene and HCN, Adiponitrile Operating Costs & Plant Construction Costs - INTRATEC