Technology History
The direct hydrocyanation of butadiene to adiponitrile (ADN) was pioneered by William C. Drinkard at DuPont in 1967, representing a breakthrough in homogeneous catalysis that revolutionized ADN production[1][2]. This technology replaced earlier multi-step chlorocyanation routes and became commercially operational in 1971 at DuPont's Orange, Texas facility, marking the 50th anniversary of commercial ADN production in 2021[3].
The process evolved from initial monodentate phosphite ligand systems to advanced bidentate phosphite ligand catalysts that offer superior performance in terms of selectivity, turnover rates, and catalyst stability[4]. In 2012, INVISTA unveiled a transformative butadiene-based "new ADN technology" that achieved improved product yields, reduced energy consumption, lower CO2 emissions, as well as virtually eliminating benzene from the production process[5].
Technology Summary and Recent Technological Improvements
Core Process Chemistry
The INVISTA process employs a three-step hydrocyanation sequence (Fig. 1)[6][7][8][9]:
- Primary Hydrocyanation: 1,3-butadiene + hydrogen cyanaide (HCN) → mixture of 3-pentenenitrile (3PN) and 2-methyl-3-butenenitrile (2M3BN)
- Isomerization: 2M3BN → 3PN (to maximize linear nitrile formation)
- Secondary Hydrocyanation: 3PN + HCN → adiponitrile (+ minor 2-methylglutaronitrile)
Figure 1 - Hydrocyanation of 1,3-butadiene to adiponitrile[7]

Recent Technological Improvements (2014 onwards)
Based on INVISTA's patent portfolio and commercial deployments[5][9][10][11][12]:
- Benzene Elimination: Complete elimination of benzene formation as a by-product
- Enhanced Process Stability: More consistent operation and reliability
- Improved Yields: Higher conversion efficiency and product selectivity
- Reduced Energy Consumption: Better heat integration and process optimization
- Lower Greenhouse Gas Emissions: Environmental performance improvements
- Reduced Capital Intensity: Lower infrastructure requirements for new installations
Detailed Step-by-Step Technology Description
Process Flow Based on Patent Analysis
Figure 2 - Hydrocyanation of 1,3-butadiene to adiponitrile block flow diagram reconstructed based on patents US9040735B2[13], US10035756B2[14], EP1344770A1[15], and related INVISTA filings
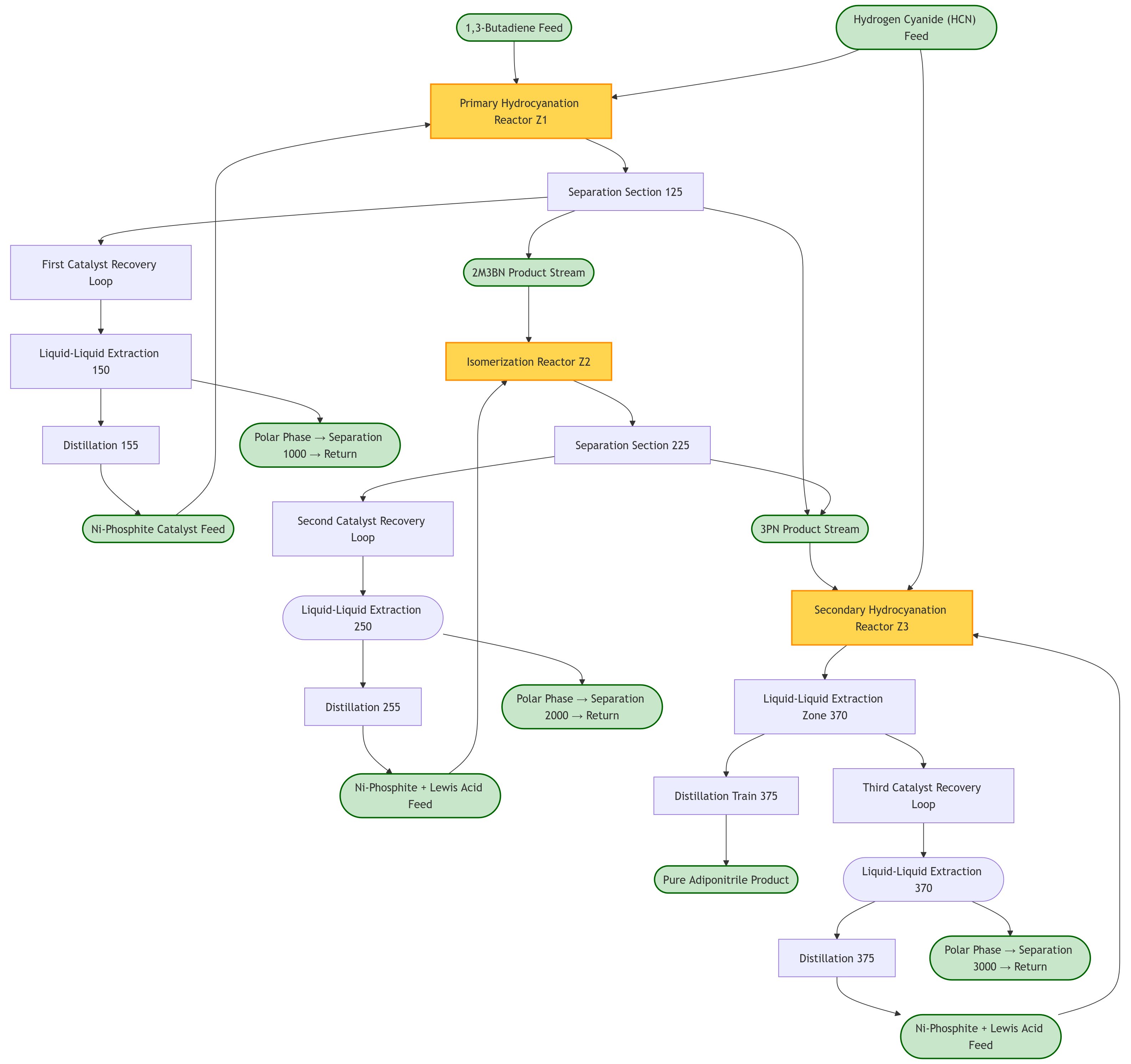
Step 1: Primary Hydrocyanation Reactor
Patent Basis: EP1344770A1[15], US20080015378A1[16]
- Reactants: 1,3-butadiene (<5 ppm 4-tert-butylcatechol) + HCN
- Catalyst System:
- Zero-valent nickel [Ni(COD)₂ preferred]
- Bidentate phosphite ligands with specific structural formulae
- Molar ratio butadiene:catalyst = 10:1 to 100,000:1 (preferred 100:1 to 5,000:1)
- Molar ratio HCN:catalyst = 10:1 to 100,000:1 (preferred 100:1 to 5,000:1)
- Conditions:
- Temperature: 25-200°C (preferred 50-150°C)
- Pressure: 5 kPa to 10,000 kPa (preferred 500-2000 kPa)
- Reaction time: 2 seconds to 24 hours
- Products: ~65% 3PN, ~35% 2M3BN mixture
Step 2: Isomerization Reactor
Patent Basis: US9040735B2[13], research insights
- Feed: 2M3BN from primary hydrocyanation
- Catalyst: Same nickel-phosphite complex + Lewis acid co-catalyst
- Lewis Acid: ZnCl₂, AlCl₃, or other Lewis acids
- Conditions:
- Temperature: 60-120°C (typical 80-120°C)
- Pressure: 1-10 bar
- Mechanism: Reversible C-CN bond activation via σ-alkyl and π-allyl intermediates
- Performance: Single-pass conversion 26.4%, selectivity to 3PN: 79.8%
Step 3: Secondary Hydrocyanation Reactor
Patent Basis: US20120035387A1[17], US9040735B2[13]
- Feed: 3PN + 4PN from Step 1 and Step 2 + HCN
- Catalyst: Ni-phosphite complex + Lewis acid promoter (preferably ZnCl₂ or AlCl₃)
- Enhanced Water Control (US9040735B2): Water concentration maintained at 100-400 ppm to prevent ligand degradation
- Conditions:
- Temperature: 25-80°C (typical 30-130°C)
- Pressure: 1-20 bar
- Residence time: 5-40 hours (typical 15-30 hours)
- HCN conversion: >99%
Step 4: Catalyst Recovery and Product Purification
Patent Basis: US10035756B2[14]
- Liquid-Liquid Extraction: Enhanced with Lewis base additives (water, ammonia, polyamines)
- Lewis Base Enhancement: Addition of bis-hexamethylenediamine (BHMT) or hexamethylenediamine (HMD) improves catalyst recovery efficiency
- Multi-stage Countercurrent Extraction: Cyclohexane as extraction solvent
- Distillation Train: Four-column system for ADN purification
Process Conditions and Parameters Summary
| Process Step |
Temperature (°C) |
Pressure (bar) |
Key Parameters |
| Primary HCN |
50-150 |
5-20 |
Butadiene:HCN:Cat
1000:1000:1 |
| Isomerization |
80-120 |
1-10 |
Ni:Lewis Acid = 1:1,
residence time variable |
| Secondary HCN |
30-130 |
1-20 |
HCN:Cat = 500-1000:1,
water 100-400 ppm |
| Extraction |
40-80 |
~1 |
Cyclohexane solvent,
polyamine additives |
Technology Performance
Yields and Selectivity
Based on patent examples and literature data[6][9]:
- Overall ADN Yield: 97-99% of theoretical
- Primary HCN Selectivity: 3PN ~65%, 2M3BN ~35%
- Isomerization Efficiency: 79.8% selectivity to 3PN
- Secondary HCN Selectivity: 81-90% to ADN (depends on Lewis acid)
- Product Purity: >99.5% ADN after distillation
Energy Efficiency
Recent INVISTA improvements[16]:
- Reduced energy consumption through advanced heat integration
- Enhanced process stability reducing energy losses
- Optimized catalyst recovery reducing regeneration energy
Economic Performance
While specific cost breakdowns remain proprietary, the process economics are driven by:
- Raw Material Efficiency: >97% conversion of butadiene and HCN[13][15][18]
- Catalyst Costs: Significant but offset by high recycling efficiency and long catalyst life[13][14][19]
- Energy Costs: Reduced through process intensification[10][11][12]
- Capital Efficiency: Improved through INVISTA's latest technology reducing capital intensity[10][11][12][18]
Commercial Experience
Global Deployment Timeline
- 1971: First commercial plant (Orange, Texas)[3]
- 2014: "New ADN technology" deployed at Orange, Texas[20]
- 2017: Technology retrofit at Butachimie JV (France)[10][12]
- 2021: Installation at Victoria, Texas ($250M investment)[21]
- 2022: Deployment at Shanghai, China (400,000 t/y capacity)[21]
- 2024: ADN production at Orange, Texas, shut down[23]
Market Position
- INVISTA: Dominant technology licensor with >90% of global ADN capacity using hydrocyanation
- Major Producers: INVISTA, Butachimie (INVISTA/Solvay JV), BASF SE
- Technology Licensing: INVISTA and Butachimie are primary licensors for new plants
- Competitive Advantage: Proprietary catalyst formulations and process optimization provide sustained market leadership[18]
Proprietary Technology Elements
Based on the patent analysis, INVISTA's competitive advantages include[13][14][15][16][17]:
- Catalyst Systems: Proprietary bidentate phosphite ligand formulations
- Process Integration: Advanced heat integration and separation technologies
- Lewis Acid Optimization: Specific promoter combinations for enhanced selectivity
- Water Management: Critical water concentration control for catalyst stability
- Extraction Enhancement: Polyamine additives for improved catalyst recovery
- Benzene-Free Innovation: undisclosed process modifications virtually eliminating benzene formation
References
- William C. Drinkard - Wikipedia
- William C Drinkard, Richard V Lindsey Jr, Hydrocyanation of olefins using selected nickel phosphite catalysts, United States Patent US3496215A, Patent Filed: Nov 11, 1965, EI Du Pont de Nemours and Co
- Nov 1987 - Health and Environmental Effects Document for Adiponitrile - U.S. Environmental Protection Agency (EPA)
- Ronald James Mckinney, Process for the hydrocyanation of butadiene, European Patent EP1344770A1, Invista Technologies Saerl
- PlasticsToday Staff, May 18, 2012 - Invista unveils new production technology for key nylon intermediate - PLASTICS TODAY
- Xuan Luo et al., Production technology of adiponitrile, E3S Web of Conferences,Vol. 441, Article Nr. 01019, (2023), DOI: 10.1051/e3sconf/202344101019
- Kaikai Liu, Research on Catalysts for the Hydrocyanation of 1,3-Butadiene to Adiponitrile, 2020 Virtual AIChE Annual Meeting, ISBN 978-0-8169-1114-1
- Liu, Kaikai, Shuai Zhang, and Minghan Han. 2020. Mechanistic Investigation on Hydrocyanation of Butadiene: A DFT Study. Catalysts 10, no. 8: 818. DOI: 10.3390/catal10080818
- Laura Bini. (2009). Mechanistic insights into the hydrocyanation reaction. [Phd Thesis 1 (Research TU/e / Graduation TU/e), Chemical Engineering and Chemistry]. Technische Universiteit Eindhoven. DOI: 10.6100/IR644067
- Sep 27, 2017 - Invista to Install New ADN Technology in France - CHEManager
- TORZEN® Nylon 6,6 Engineering Polymers Brochure - STUDYLIB
- Mary Bailey, Sep 21, 2017 - Invista to install new adiponitrile process technology at Butachimie site in France - Chemical Engineering
- Aki Sudhir et al., Process for making nitriles, United States Patent US9040735B2, Patent filed: Oct 4, 2012, Invista North America S.a.r.l.
- William J. Tenn, Integrated process for nitrile manufacture with enhanced liquid-liquid extraction, United States Patent US10035756B2, Patent filed: Jun 25, 2015, Invista North America S.a.r.l.
- Ronald James McKinney, Process for the hydrocyanation of butadiene, European Patent EP1344770A1, Patent filed: Mar 10, 2003, E.I. du Pont de Nemours and Company
- Thomas Foo et al., Process for making 3-pentenenitrile by hydrocyanation of butadiene, United States Patent US20080015378A1, Patent filed: Jul 12, 2007, Invista North America LLC
- James Michael Gartner et al., Hydrocyanation of 2-pentenenitrile, United States Patent US20120035387A1, Patent filed Feb 7, 2011, Invista North America S.a.r.l.
- Q3 2024 - Adiponitrile Production from Butadiene and HCN, Adiponitrile Operating Costs & Plant Construction Costs - INTRATEC
- Laura Bini et al. Ligand development in the Ni-catalyzed hydrocyanation of alkenes. Chemical Communications, vol. 46, no. 44, 2010, pp. 8325–8334. The Royal Society of Chemistry. DOI: 10.1039/C0CC01452D
- Dec 17, 2018 - INVISTA’s Orange, Texas, Site Sets Adiponitrile Production Record - Defence Industries
- Mar 26, 2021 - Invista Concludes Technology Upgrade at Victoria ADN Plant - Process Worldwide
- Nov 25, 2022 - INVISTA Celebrates Inauguration of 400,000-ton/year ADN Plant at the Shanghai Chemical Industry Park - INVISTA
- Oct 10, 2023 - INVISTA Closes Texas Facility, Amplifying Its Strategic Commitment to Shanghai - China Chemical Market Insights












