Historical Development
The phosgenation route to isocyanates was first developed in the early 20th century and became the dominant commercial method by the 1940s. Key historical milestones include:
- 1930s-1940s: Initial commercial development of liquid-phase phosgenation processes by IG Farben (Germany), with Werner Siefken's 1936 breakthrough establishing the foundation for industrial isocyanate production
- 1950s-1960s: Scale-up to large industrial production for TDI and MDI by Bayer, BASF (Germany), DuPont, Mobay Chemical, Dow Chemical, and Union Carbide (USA)
- 1980s: Development of gas-phase phosgenation technology by Bayer (now Covestro), offering significant energy savings for volatile amine applications
- 1990s-2000s: Process intensification and environmental improvements led by BASF, Covestro, Huntsman, and Dow, with technology licensing expansion to Asian markets
- 2010s-Present: Advanced process control, energy efficiency improvements, and alternative route research, with market leadership shifting to Wanhua Chemical (China) while BASF and Covestro maintain technology leadership through continued innovation and global licensing
Process Summary and Chemistry
Core Chemistry
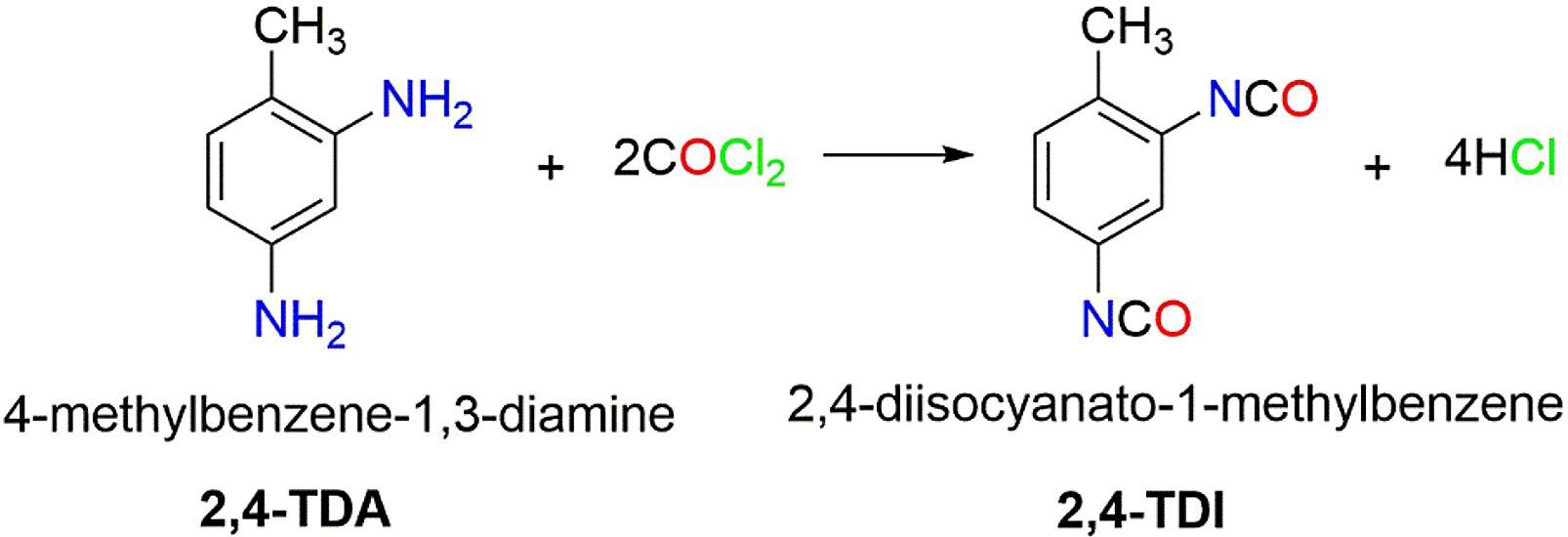
The phosgenation process involves the reaction of organic amines with phosgene to produce isocyanates via a two-step mechanism (example of a difunctional amine):
Step 1: Carbamoyl Chloride Formation
H2N—R—NH2 + 2 O=CCl2 → Cl—C(=O)—NH—R—NH—C(=O)—Cl + 2 H—Cl
Step 2: Thermolysis to Isocyanate
Cl—C(=O)—NH—R—NH—C(=O)—Cl → O=C=N—R—N=C=O + 2 H—Cl
Overall Reaction for Diisocyanates:
H2N—R—NH2 + 2 O=CCl2 → O=C=N—R—N=C=O + 4 H—Cl
Figure 1 - TDA reaction with phosgene to form TDI[7]
Process Types and Applicability
Modern isocyanate production employs two main phosgenation approaches with different applicability ranges:
Liquid-Phase Process (Historical): Universal applicability to all amines
- Operating Temperature: TDI: 65-95°C, MDI: ~200°C, HDI: 130-170°C (optimum 150°C)
- Operating Pressure: 1-5 bar gauge (typically 1-3 bar)
- Applicability: All amine types - aromatic, aliphatic, alicyclic, specialty amines
- Solvents: Monochlorobenzene, orthodichlorobenzene, toluene
Gas-Phase Process: Limited to volatile amines only
- Operating conditions: 300-400°C (typically ~320°C practical operation)
- Applicability: Restricted to volatile amines that can be vaporized without decomposition
- Successfully applied: TDA (for TDI), select aliphatic diamines like HDA (for HDI)
- Not suitable: High molecular weight diamines, thermally unstable amines, complex aromatic diamines
Figure 2 - Conceptual description of the amine phosgenation process
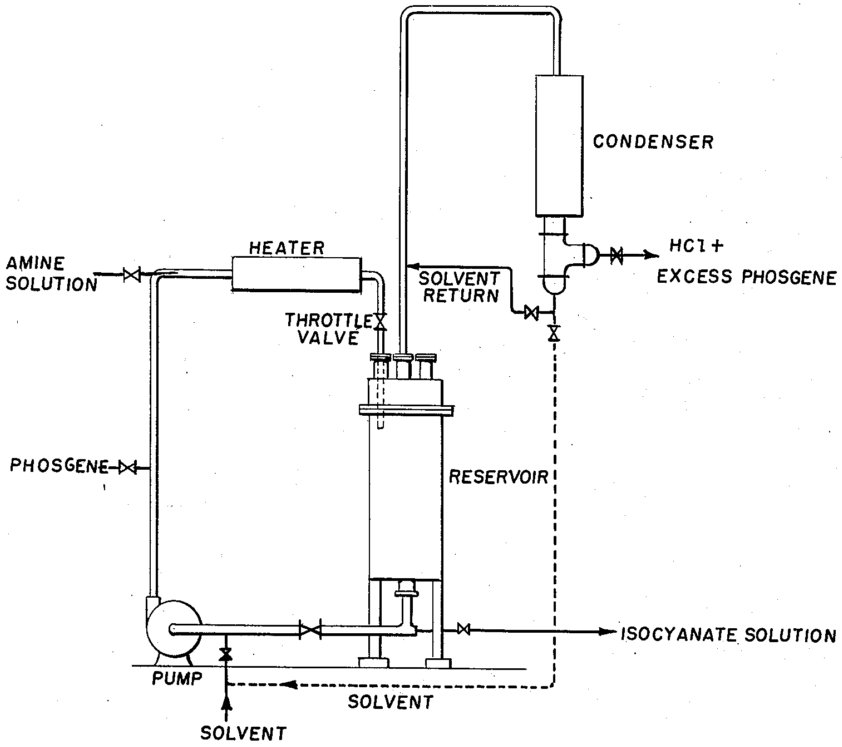
Detailed Process Description with Practical Operating Conditions
Liquid-Phase Phosgenation Process
Reactor Systems
- Reactor Type: Stirred tank reactors with agitation systems
- Configuration: Single-stage or multi-stage continuous tanks
- Temperature Control: Steam-circulating jackets for precise temperature management
- Pressure: Standard vessels operating 1-5 bar gauge
- Residence Time: Several hours (3-15 hours typical)
Figure 3 - Process for the manufacture of organic isocyanates in liquid phase[5]
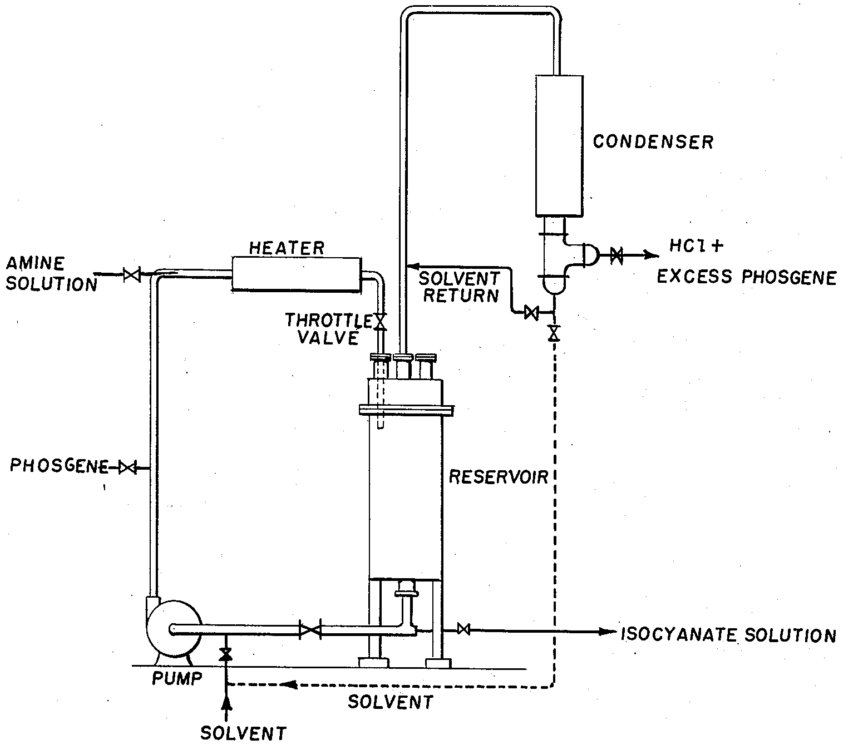
Specialized Equipment
- Salt-forming reactors: For amine hydrochloride preparation (HDI process)
- Agitating blades: High-speed mixing (2.5-6 m/sec circumferential velocity)
- Feed systems: Dual nozzle systems for simultaneous amine/HCl introduction
- Recovery systems: Conventional condensers and gas-liquid separators
Practical Operating Conditions (Based on Patent Examples)
- TDI Production: 65-80°C for carbamoyl chloride formation, hot phosgenation to ~90°C
- MDI Production: ~200°C for phosgenation with 6-8 molar excess phosgene
- HDI Production: 130-170°C (optimum 150°C), 1-3 kg/cm²G pressure
- Residence Time: 3.3-15 hours depending on amine type and conversion requirements
Reactor Configuration Examples
- Two-stage HDI process: First stage 3.3 hours (70-95% conversion), second stage 6.9 hours (>99% conversion)
- Pressure optimization: 2 kg/cm²G optimal for phosgene recovery vs. by-product formation
- Phosgene flow rate: 5-12 molar times per hour the total HDA·HCl + HDI content
Gas-Phase Phosgenation Process
Reactor Systems
- Reactor Type: Specialized high-temperature tubular reactors
- Configuration: Short residence time reactors with rapid mixing nozzles
- Temperature Control: High-temperature heating systems (up to 400°C capability)
- Pressure: Atmospheric to slightly elevated pressure
- Residence Time: <1 minute (vs. hours for liquid phase)
Figure 4 - Production of TDI by the gas phase phosgene method[6]
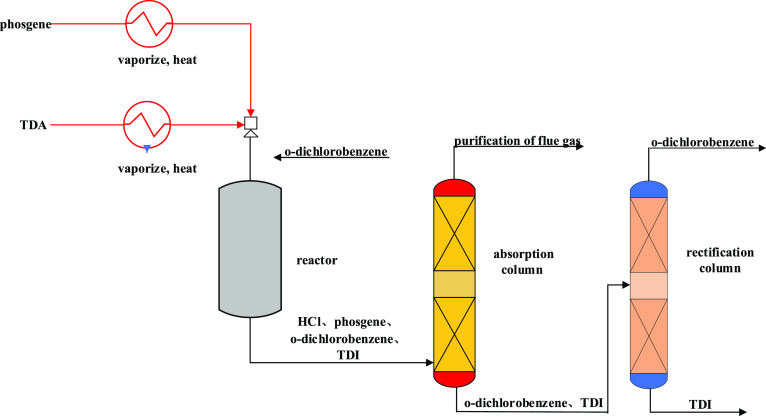
Specialized Equipment
- Feed vaporization: High-temperature evaporators for amine and phosgene
- Rapid mixing: Specialized reactor nozzles for instantaneous gas-phase contact
- Quench systems: Immediate cooling with solvent spray to prevent side reactions
- Heat integration: Advanced heat recovery systems for energy efficiency
Practical Operating Conditions (Based on Commercial Examples)
- TDI Production: 320°C preheating, reactor operation at 300°C, 0.1 MPa pressure
- Contact Time: <1 minute residence time in reactor
- Phosgene Ratio: High molar ratios to maintain reaction kinetics in gas phase
- Quench Temperature: Immediate cooling to prevent product decomposition
Gas-Phase Process Limitations and applicability
- Maximum Operating Temperature: ~400°C before most amines decompose
- Challenging Applications: MDI, PMDI due to low amine volatilty, viscosity challenges, thermal sensitivity associated with decomposition risks
- TDI production: Primary commercial success with proven large-scale operation
- Select HDI production: Where thermal stability permits high-temperature operation
- Economic drivers: Large-scale plants where energy savings justify technology complexity
Commercial Performance
- Covestro Dormagen TDI plant: 300,000 t/y capacity using gas-phase technology
- Energy Savings: 60% energy reduction vs. liquid-phase process
- Solvent Reduction: 80% less solvent requirement
- Investment Savings: 20% lower capital costs for new plants
Process Efficiency
Conversion and Yields
- Liquid Phase: 85-95% amine conversion typically achieved, 96% HDI yield demonstrated
- Gas Phase: Up to 99% theoretical yields possible for suitable amines
- Overall Efficiency: Modern processes achieve >90% carbon efficiency across both technologies
Energy and Environmental Performance
- Gas-Phase Benefits: 60% energy reduction, 22,000 tons CO2/year savings (300,000 t/y plant)
- Liquid-Phase Advantages: Universal applicability, established technology with proven reliability
- Solvent Management: Gas-phase 80% solvent reduction vs. liquid-phase comprehensive solvent recovery
Process Economics
Capital Investment
- Liquid Phase: Standard equipment costs, established supply chains, higher solvent system costs
- Gas Phase: 20% capital reduction for suitable applications, specialized high-temperature equipment
- Scale Economics: >200,000 t/y plants required for competitive gas-phase implementation
Operating Costs
- Energy: Gas-phase significant advantage through process intensification
- Raw Materials: 40% phosgene savings per kg TDI in gas-phase process
- Solvent: Liquid-phase higher solvent costs, gas-phase reduced handling costs
- Maintenance: Liquid-phase conventional maintenance, gas-phase specialized high-temperature equipment
Isocyanate Industry: Producers and Technology Providers
| Company |
Primary
Products |
Production Capacity (kt/y) |
Technology/
Process |
Wanhua
Chemical Group |
MDI, TDI,
HDI, ADI* |
4,200 (MDI) |
Liquid Phase,
Advanced Integration |
| BASF SE |
MDI, TDI, Specialty |
2,020 (MDI) |
Liquid + Gas Phase, Licensing |
| Covestro AG |
MDI, TDI,
HDI, IPDI |
1,770 (MDI) |
Gas Phase Pioneer, Licensing |
| Huntsman Corporation |
MDI
Focus |
1,370 (MDI) |
Liquid Phase MDI Specialization |
| Dow Inc. |
TDI, MDI |
1,110 (MDI) |
Liquid Phase, Integrated Operations |
| Kumho Mitsui Chemicals |
MDI |
610 (MDI) |
Mitsui Technology License |
Sadara
Chemical |
TDI, MDI |
450 (TDI+MDI) |
SABIC JV
Technology |
Shanghai
SECCO |
MDI |
300 (MDI) |
Integrated PetChem Complex |
| Tosoh Corporation |
TDI, MDI |
300 (TDI+MDI) |
Integrated Vinyl
Chain Technology |
OCI
Co. Ltd. |
TDI |
250 (TDI) |
TDI Focus,
Liquid Phase |
Evonik
Industries |
IPDI, Specialty Isocyanates |
200 (IPDI) |
Specialty Aliphatic Technologies |
| Vencorex |
HDI, IPDI |
180 (HDI) |
HDI Specialization Technology |
Mitsui
Chemicals Inc. |
TDI, XDI**, Specialty |
50 (TDI, optimized) |
Proprietary TDI/XDI Technology |
| DuPont |
TDI
(Historical) |
Discontinued |
Historical
TDI Production |
| Sumitomo Chemical |
TDI
(Historical JV) |
Discontinued (2004) |
Former Bayer
Joint Venture |
*ADI: Aliphatic Diisocyanates; **XDI: Xylylene Diisocyanate.
Summary Statistics
- Total Active Major Producers: 13 companies
- Combined MDI Capacity: ~12,130 kt/y
- Market Leaders: Top 5 producers control ~85% of global capacity
- Technology Distribution: Mix of liquid-phase (dominant) and gas-phase processes
- Geographic Distribution: Asia-Pacific leads with 60% of global capacity
Key Technology Providers
- Gas-Phase Technology: Covestro (pioneer), BASF
- Liquid-Phase Technology: Most producers use proven liquid-phase processes
- Specialty Technologies: Evonik (IPDI), Vencorex (HDI), Mitsui (integrated systems)
- Licensing Leaders: BASF, Covestro, Mitsui Chemicals for comprehensive technology packages
References
- Encyclopedia.com. Bayer, Otto Georg Wilhelm.
- Chiang Mai Foam Factory. 2025. History of Polyurethane.
- BASF. 2019. TDI Handbook North America.
- BASF. 2019. MDI Handbook North America.
- T.R. Beck et al. United States patent US2822373: Preparation of derivatives of isocyanic acid by reaction of amines with carbonyl halides, e.g. with phosgene. Priority date: Mar 15, 1954. Assignee: E.I. du Pont de Nemours and Company.
- Guo Z, Ding X, Wang Y. How To Get Isocyanate? ACS Omega. 2024 Feb 28;9(10):11168-11180. DOI: 10.1021/acsomega.3c10069. PMID: 38496933; PMCID: PMC10938423.
- Thangaraj R, Horváth T, Boros RZ, Viskolcz B, Szori M. A Theoretical Study on the Phosgenation of 2,4-Toluenediamine (2,4-TDA). Polymers (Basel). 2022 May 31;14(11):2254. DOI: 10.3390/polym14112254. PMID: 35683928; PMCID: PMC9182759.
- T. A. Ryan et al. Phosgene. 4 Industrial manufacture and uses. Topics in Inorganic and General Chemistry, Pergamon, Volume 24, 1996, Pages 167-221, ISSN 0082-495X, ISBN 9780444824455, DOI 10.1016/S0082-495X(07)80009-6.
- Conventional Production of TDI. Ebrary.net.
- Production of MDI. Ebrary.net.
- Production of Aliphatic Isocyanates. Ebrary.net.
- M. Böhm et al. United States patent US7504533B2: Process for the production of isocyanates. Priority date: Apr 24, 2006. Assignee: Covestro Deutschland AG, Covestro LLC.
- Xin Feng et al. Chinese patent CN101623615A: Sleeve distributed gas phase phosgenation reactor and method for synthesizing isocyanate. Priority date Aug 11, 2009. Assignee: Tianjin University, Sedin Engineering Co Ltd.
- Covestro. Gas phase technology: 60% less energy consumption.
- Ajioka et al. United States patent US4922005: Process for preparing hexamethylene diisocyanate. Priority date Nov 13, 205. Assignee: Mitsui Toatsu Chemicals, Incorporated.
- R. Swan. Jul 11, 2025. How Toluene Diisocyanate (TDI) is made: A Quick Dive into Process & Sustainability. ChemAnalyst.
- Covestro. Mar 20, 2025. Press release: Covestro Successfully Completes Modernization of its Dormagen TDI Plant.
- ECHEMI. Dec 24, 2024. Chongqing's Key Projects Investment Reaches 450.9 Billion Yuan in 2024; BASF's MDI Capacity Increased to 530,000 Tons.
- ECHEMI. Aug 27, 2024. MDI Market Storm! Supply Gap Exceeds 2 Million Tons, Price Surges 22%! Multiple Giants Suspend Production for Maintenance, Impact May Last Until 2025.
- S. Kothrade. Sep 2024. BASF Capital Markets Day 2024. BASF.
- C. Dong-hun, H. Yubin. Apr 4, 2025. Kumho Mitsui Chemical expands MDI production. Pulseby Maeil Business News Korea.
- Mitsui Chemicals. Oct 23, 2024. News release: Kumho Mitsui Chemicals Begins Operations at New MDI Production Facilities.
- KMCI. Overview.
- ECHEMI. Dec 30, 2021. Brief Analysis of MDI, TDI, ADI Industry.
- F. Christensen et al. Environmental Project No. 1537, 2014: Survey of certain isocyanates (MDI and TDI), part of the LOUS-review. ISBN: 978-87-93026-91-9. The Danish Environmental Protection Agency.
- Plastics Today. Jan 1, 2024. PUR projects taking shape in China.
- Sumitomo Chemical. Jun 1, 2022. Sumitomo Chemical IR Day 2022 Spring.