Technology

- Name
- Axens MTBE/ETBE
- Owner
-
/ Axens SA - Brand
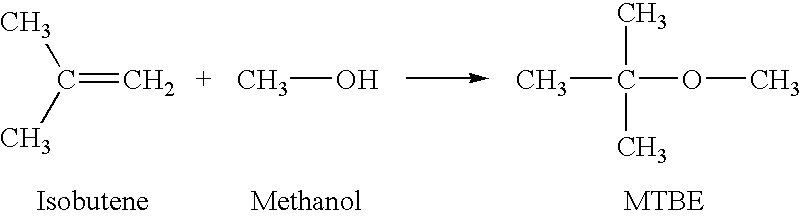
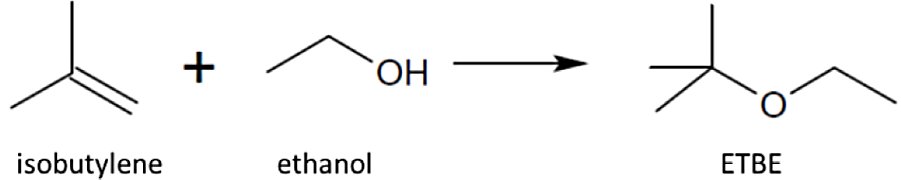
- Process
- Etherification
- Type
- Etherification of Isoolefins
- Available
-

- #TE350
Description
Your insights will be shown here
| Entity | Site (Country) | Asset (Plant) | |||
|---|---|---|---|---|---|

|

|
|
MTBE Plant | ||

|

|
|
MTBE Plant | ||

|

|
|
MTBE Unit |
Content provided by
| Transaction | Name | Date |
|---|---|---|
| Modified by |
|
7/17/2025 10:04 AM |
| Added by |
|
7/17/2025 8:57 AM |