Butadienes are a group of organic compounds with the molecular formula C₄H₆, characterized by the presence of two double bonds. The two main isomers of butadiene are 1,3-butadiene and 1,2-butadiene, which differ in the arrangement of their double bonds.
1,3-Butadiene
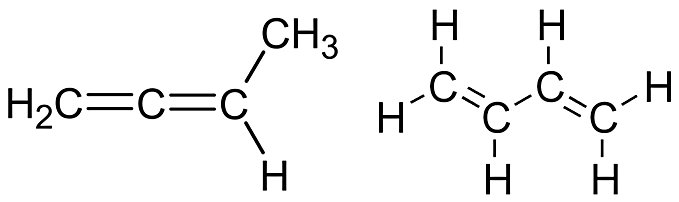
1,3-Butadiene is a conjugated diene, meaning its double bonds are separated by a single bond, allowing for resonance stabilization. Its structure is CH₂=CH−CH=CH₂. This compound is widely used in industrial applications, particularly in the production of synthetic rubbers and polymers like styrene-butadiene rubber (used in tires) and ABS plastics. It is a colorless, flammable gas with a mild aromatic odor and is thermodynamically stabilized due to conjugation effects. The most stable conformation of 1,3-butadiene is the s-trans form, where the molecule adopts a planar geometry to maximize conjugation.
1,2-Butadiene
1,2-Butadiene is an allene (a cumulated diene), meaning its two double bonds are adjacent. Its structure is CH₂=C=CH−CH₃. Unlike 1,3-butadiene, it does not exhibit resonance stabilization and has limited industrial applications. It is less stable thermodynamically and lacks the extensive use seen with 1,3-butadiene.
Comparison of Isomers
| Property |
1,3-Butadiene |
1,2-Butadiene |
| Structure |
CH₂=CH−CH=CH₂ |
CH₂=C=CH−CH₃ |
| Type of Diene |
Conjugated |
Cumulated (Allene) |
| Stability |
Thermodynamically stable |
Less stable |
| Industrial Use |
Synthetic rubber, plastics |
Limited |
| Conformation |
s-trans (most stable) |
No significant conformations |
Both isomers are flammable gases and must be handled carefully due to their explosive potential when mixed with air.
Reference
A.I. generated description