Main-Product
- Product
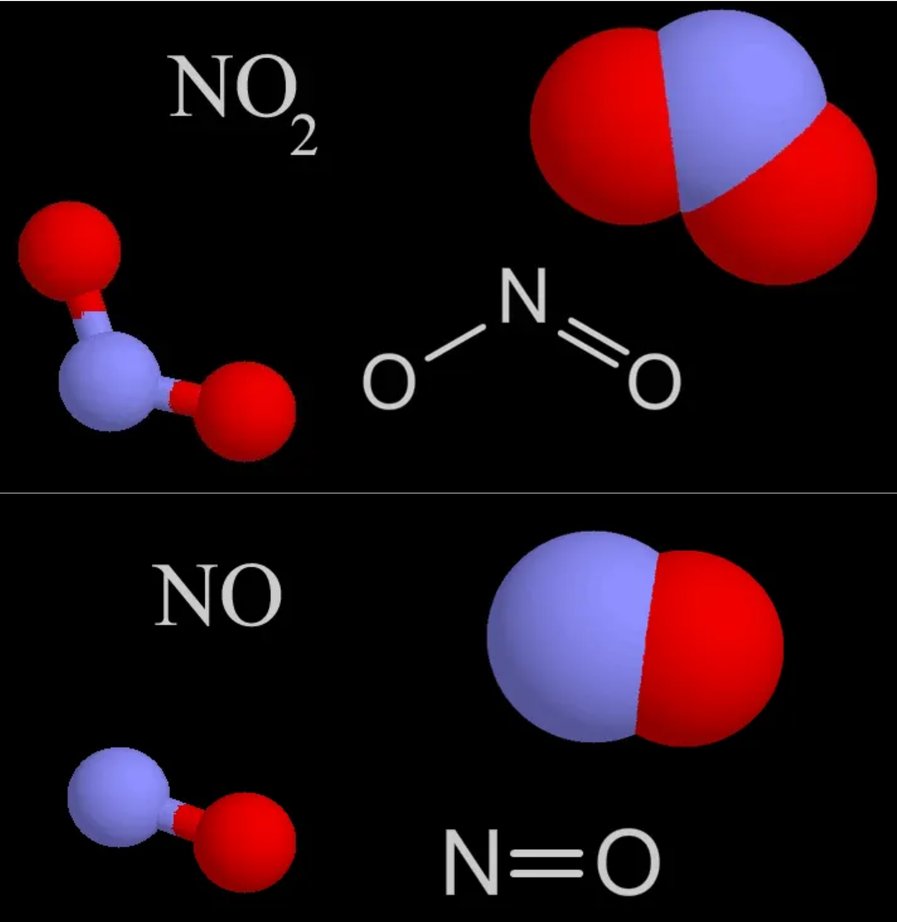
- Nitrogen Oxides
Product Categories, Description and Properties
- Segment
- Refined Products
- Main-Family
- Refinery Gases
- Sub-Family
- Gaseous nitrogen compounds
- Link
Description
Your insights will be shown here
Properties
- Status
- A
- Unit of Measure
- Metric Ton
- Physical State
-
Gas
System Info
- Update by
-
 Kokel, Nicolas
Kokel, Nicolas - Last Update
- 1/1/2025 8:49 PM
- Added
- 1/1/2025 8:23 PM
Product Communicator
(*=Default)| Product | Title | Date | |
|---|---|---|---|

|
1/1/2025 |
| Products (Quick Access) | Abbr. | Default |
|---|---|---|
| Nitrogen Oxides | NOx | |
| Nitrogen Dioxide | NO₂ | |
| Nitrous Oxide | N2O |