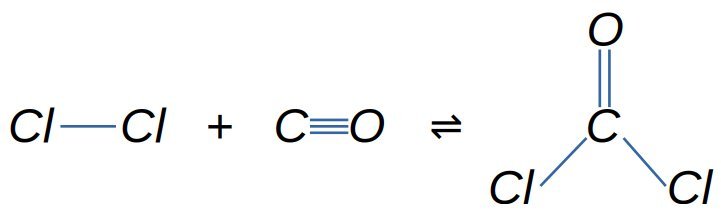Technology Type
- Type
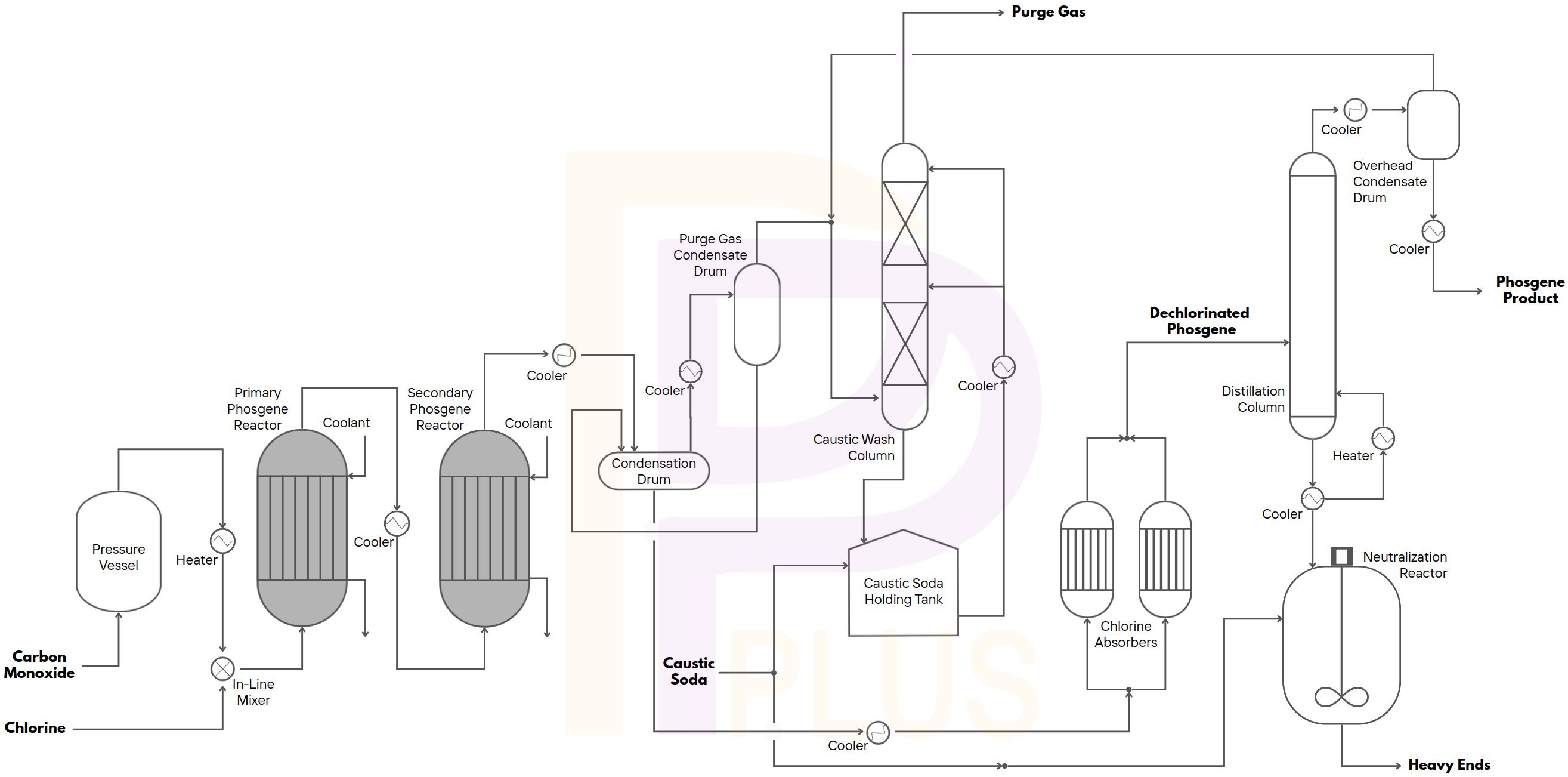
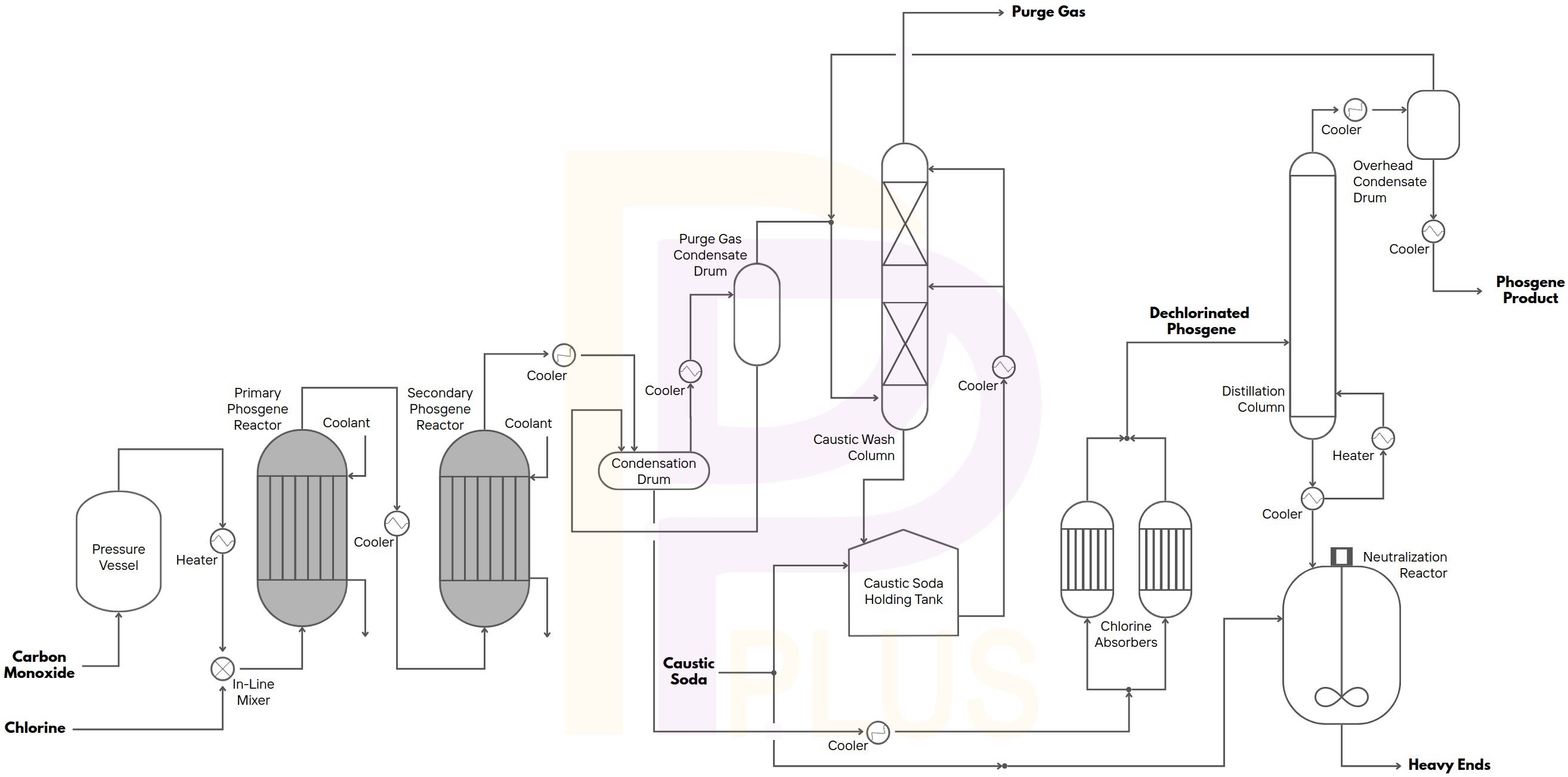
- Phosgene from Chlorine and Carbon Monoxide
- Process
- Carbonylation
-

- #TT61
Description
Your insights will be shown here
| Technology | Owner | Entity |
|---|---|---|
| Technology | Technology Entity | |

|
Mitsui Chemicals |
Content provided by
| Transaction | Name | Date |
|---|---|---|
| Modified by |
|
9/6/2025 10:54 AM |
| Added | 4/2/2022 5:18 PM |