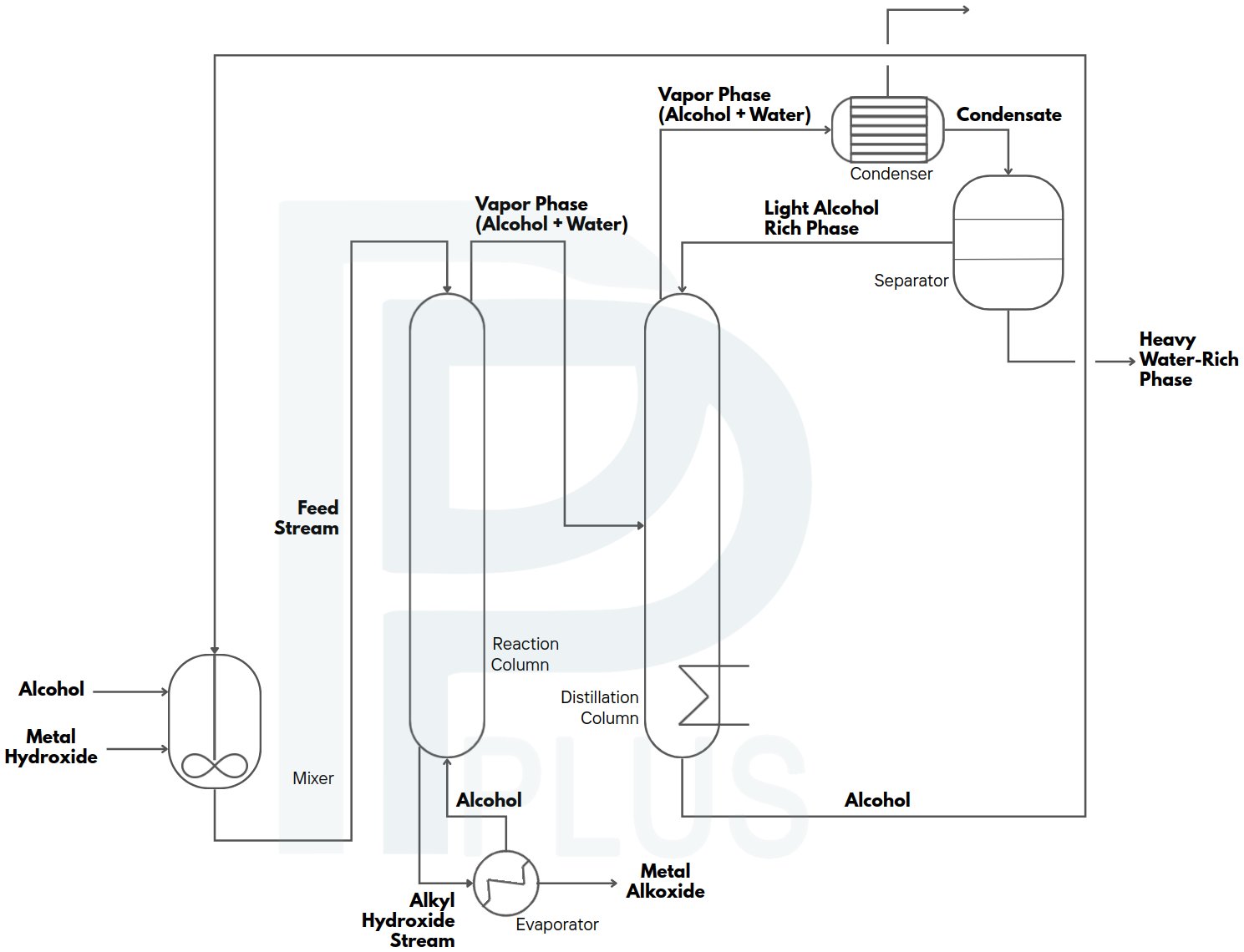
Alkoxides are organometallic compounds formed by the reaction of alkali metal hydroxides with alcohols, creating metal-oxygen-carbon bonds (M-OR). In industrial contexts, alkoxide production focuses on alkali metal alkoxides such as sodium methoxide (NaOCH3) and potassium methoxide (KOCH3), which are critical catalysts for biodiesel production, pharmaceutical synthesis, and specialty chemical manufacturing. The dominant industrial route employs sodium hydroxide (NaOH) or potassium hydroxide (KOH) reacting with methanol.
Core Chemistry and Reaction Mechanisms
Primary Industrial Reaction:
The alkali hydroxide-methanol route represents the foundation of commercial alkoxide production, based on the following equilibrium reactions:
For Sodium Methoxide:
NaOH + CH3OH → NaOCH3 + H2O ΔH = -15.2 kJ/mol
For Potassium Methoxide:
KOH + CH3OH → KOCH3 + H2O ΔH = -18.7 kJ/mol
Thermodynamic Challenge:
The fundamental engineering challenge lies in the unfavorable equilibrium position. The equilibrium constant for these reactions is relatively small (K ≈ 0.1-0.3 at 100°C), meaning:
- Water formation drives equilibrium backward toward reactants
- Only 10-30% conversion achievable without water removal
- Continuous water separation is essential for high conversion
Advantages of Potassium vs. Sodium Route
Potassium hydroxide demonstrates superior performance characteristics:
- Higher dissolution rate in methanol compared to sodium hydroxide
- Better mass transfer due to enhanced solubility
- Lower viscosity of reaction mixture improves heat and mass transfer
- Higher reaction rates under equivalent conditions
Industrial Process Engineering
Reactive Distillation Technology
Modern alkoxide plants employ reactive distillation columns that simultaneously perform reaction and separation. This integrated approach offers several advantages:
Process Configuration:
- Reaction zone: Middle section packed with catalyst or containing dissolved hydroxide
- Rectifying section: Upper section for methanol-water separation
- Stripping section: Lower section for product concentration
- Continuous operation: Steady-state feeds and product withdrawal
Operating Conditions:
- Temperature: 120-175°C in reaction zone
- Pressure: 0.3-2.5 MPa depending on desired boiling point control
- Residence time: 15-45 minutes in reactive zone
- Reflux ratio: 2-5:1 for effective water separation
Water Removal Technology - The Critical Challenge
Azeotropic Behavior
The methanol-water system forms a minimum boiling azeotrope at 64.7°C containing 12.1 mol% water. This azeotropic behavior complicates direct separation and requires specialized techniques:
Azeotropic Distillation Methods:
- Pressure swing distillation: Exploits pressure dependence of azeotropic composition
- Extractive distillation: Uses high-boiling solvents to break azeotrope
- Heterogeneous azeotropic distillation: Employs phase separation after condensation
- Membrane pervaporation: Selective water permeation through specialized membranes
Advanced Water Separation Techniques
Modern plants integrate multiple separation technologies:
Molecular Sieves:
- Type 3A zeolites: Selective adsorption of water molecules
- Regeneration cycles: Thermal swing adsorption at 200-300°C
- Efficiency: Achieves <0.1% water content in recycled methanol
Vapor Recompression:
- Mechanical compression: Reuses latent heat from distillation vapors
- Energy savings: Reduces steam consumption by 40-60%
- Process integration: Heat pumps upgrade low-grade waste heat
Process Intensification and Advanced Configurations
Reactive Dividing-Wall Columns (R-DWC)
Advanced plants employ dividing-wall technology for enhanced efficiency:
- Simultaneous separation: Products and unreacted materials separated in single unit
- Energy reduction: 20-30% lower energy consumption vs. conventional sequences
- Capital savings: Eliminates separate rectification columns
- Higher conversion: Internal recycle increases overall conversion
Heat Integration Strategies
Modern alkoxide production emphasizes thermal efficiency:
Multi-effect Configuration:
- Heat exchangers: Preheat feeds using hot product streams
- Steam generation: Reaction exotherm produces process steam
- Waste heat recovery: Low-grade heat used for feed preheating
Process Control and Automation
Advanced plants incorporate distributed control systems (DCS):
- Temperature profiles: Maintain optimal reaction conditions throughout column
- Composition control: Real-time monitoring of water content and conversion
- Safety interlocks: Automatic shutdown for temperature/pressure excursions
- Optimization algorithms: Maximize yield while minimizing energy consumption
Process Optimization Strategies
Commercial plants focus on several optimization targets:
- Yield maximization: Advanced water removal achieves >98% conversion
- Energy minimization: Integrated heat recovery reduces operating costs
- Equipment reliability: Robust design ensures high on-stream factors
- Product quality: Consistent specifications reduce downstream processing costs
Safety Considerations and Process Hazards
Chemical Reactivity Management
Alkoxide production involves several safety challenges:
Hydroxide Handling:
- Caustic burns: Sodium and potassium hydroxide cause severe skin/eye damage
- Moisture sensitivity: Hydroxides absorb atmospheric moisture, affecting stoichiometry
- Heat generation: Dissolution in methanol is highly exothermic
Process Safety Systems
Industrial facilities incorporate multiple safety layers:
- Emergency relief: Pressure relief valves protect against overpressure
- Inert atmosphere: Nitrogen blanketing prevents moisture ingress
- Materials selection: Stainless steel construction resists caustic corrosion
- Leak detection: Continuous monitoring for hydroxide/methanol vapors
Product Quality and Purification
Commercial Specifications
Industrial alkoxide solutions must meet stringent quality requirements:
Sodium Methoxide (25-30% in methanol):
- Active content: 25.0-30.0% NaOCH3 by weight
- Water content: <0.5% maximum
- Free NaOH: <0.5% maximum
- Appearance: Clear to slightly hazy solution
Potassium Methoxide (30-32% in methanol):
- Active content: 30.0-32.0% KOCH3 by weight
- Water content: <0.3% maximum
- Free KOH: <0.3% maximum
- Color: Clear amber to colorless
Environmental and Sustainability Aspects
Process Emissions Control
Modern alkoxide plants address environmental impact:
- VOC recovery: Methanol vapor recovery systems minimize atmospheric emissions
- Wastewater treatment: Neutralization of alkaline process streams before discharge
- Energy efficiency: Heat integration reduces CO2 footprint
References
- Wikipedia. Potassium methoxide
- Ataman Chemicals. Potassium Methylate
- N. Aeamsuksai et al.. Nov. 30, 2020. Comparison of Different Synthesis Schemes for Production of Sodium Methoxide from Methanol and Sodium Hydroxide. Eng. J., Vol. 24. Issue 6. ISSN: 0125-8281. DOI:10.4186/ej.2020.24.6.63
- W. Smith. Nov 29, 2022. Write a reaction that shows what happens when methanol is treated with potassium hydroxide? Story of Mathematics
- A.A. Kiss et al.. Dec 11, 2018. Reactive distillation: Stepping up to the next level of process intensification. Industrial & Engineering Chemistry Research. DOI: 10.1021/acs.iecr.8b05450
- V. Ladnak et al.. World patent WO2016083175A1: Producing a metal alkoxide using a reactive distillation process. Priority date: Nov 16, 2015. Assignee: BASF SE
- Lai Mingkong, Zhang Hui. Chinese patent CN105884578A: Method for high temperature and high pressure production of sodium methoxide and device thereof. Priority date: Apr 21, 2016. Assignee: Individual
- J.C. Card, L.M. Farrell. Oak Ridge National Laboratory. Separation of Alcohol-Water Mixtures Using Salts. U.S. Department of Energy, Office of Scientific and Technical Information
- C. Shaji et al.. 2023. An overview of Dehydration of Ethanol by Azeotropic Distillation using various entrainer. IJRTI, Vol. 8, Issue 6. ISSN: 2456-3315. DOI: 10.1155/2022/8374471
- M. Mekala et al.. Jan 1, 2022. Water Removal from an Ethanol-Water Mixture at Azeotropic Condition by Adsorption Technique. Science & Technology, 2022. DOI: 10.1155/2022/8374471
- J. van Kampen et al.. Sep, 25, 2023. Breaking azeotropes by reactive adsorption: A case for sorption-enhanced dimethyl carbonate synthesis.Chemical Engineering Science, 282, Article 119326. DOI: 10.1016/j.ces.2023.119326
- Peng Tao, Han Dong, Xia Junjun. Chinese patent CN103288593A: Device and method for producing sodium methoxide through recompression of mechanical steam. Priority date Jun 4, 2016. Assignee: Jiangsu Leke Energy-Saving Technology Co., Ltd.
- JunYuang Petroleum Group. Mar 3, 2022. Optimization Process of Sodium Methylate
- V. Ladnak et al.. World Patent WO2016083175A1: Producing a metal alkoxide using a reactive distillation process. Priority date Nov 16, 2015. Application filed by BASF SE.