Technology History and Heritage
Evonik's alkoxides technology has evolved over 80 years of catalysts development expertise and decades of alkoxide production experience. The technology builds upon Evonik's legacy in homogeneous and heterogeneous catalysts, with alkoxide production dating back to the company's early chemical operations.
Historical Development:
- 1970s-1980s: Initial alkoxide production using conventional batch processes
- 1990s-2000s: Transition to continuous processes and reactive distillation technology
- 2007-2010: Development of energy-efficient processes with vapor recompression (US Patent 7847133B2)
- 2020-2023: Advanced technology development with simultaneous sodium/potassium production capabilities (US Patent 11746075B2)
- 2023-2025: Implementation of reduced carbon emission processes and global expansion
The technology heritage stems from Evonik's Degussa legacy, with continuous innovation in process intensification and energy efficiency.
The "SMART" (Sodium Methylate Advanced Recompression Technology) designation was introduced as the project name and technology focus for Evonik’s Singapore alkoxides plant inaugurated in 2025, highlighting the latest advances in energy-efficient alkoxide production but not serving as a universal branding for all of Evonik’s global alkoxide process technology.
Technology Summary and Chemistry
Core Technology Concept:
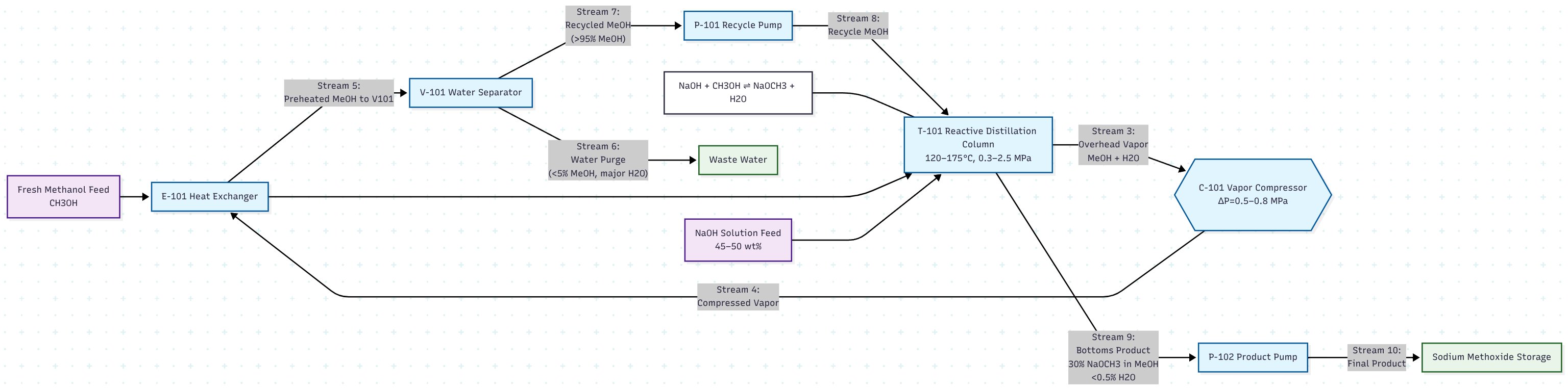
SMART technology combines reactive distillation with advanced vapor recompression to produce high-quality sodium methoxide and potassium methoxide from hydroxide feedstocks and methanol. The process addresses the fundamental thermodynamic limitation of the hydroxide-methanol equilibrium reaction through continuous water removal and energy recovery (Fig. 1):
Figure 1 - Evonik sodium methylate technology via the alkali hydroxide-methanol route (Appendix 1)
Primary Chemistry:
The technology is based on the deprotonation reactions:
- Sodium route: NaOH + CH₃OH ⇌ NaOCH₃ + H₂O (ΔH = -15.2 kJ/mol)
- Potassium route: KOH + CH₃OH ⇌ KOCH₃ + H₂O (ΔH = -18.7 kJ/mol)
Key Technological Innovations:
- Simultaneous Production: Capability to produce both sodium and potassium alkoxides in a single process unit
- Advanced Recompression: Mechanical vapor recompression reduces energy consumption by 40-60%
- Energy Integration: Heat exchanger networks maximize thermal efficiency
- Process Intensification: Reactive distillation eliminates separate reaction and separation steps
Detailed Step-by-Step Process Description
Step 1: Feed Preparation and Injection
- Methanol Feed Stream (S-1): High-purity methanol (>99.5%) is preheated to 60-80°C using recovered heat from the process
- Hydroxide Feed Stream (S-2): Aqueous NaOH solution (45-50 wt%) or KOH solution (45-48 wt%) is prepared and fed to the reactive zone
- Feed Ratio: Maintained at slight stoichiometric excess of methanol (1.05-1.10:1 molar ratio) to drive equilibrium conversion
Step 2: Reactive Distillation
- Reaction Zone (Middle Section): 8-12 theoretical stages operating at 120-175°C and 0.3-2.5 MPa
- Mass Transfer: Intimate contact between vapor and liquid phases enhances reaction kinetics
- Equilibrium Shift: Continuous water removal drives conversion to >98% of theoretical
- Residence Time: 15-45 minutes in reactive zone ensures complete conversion
Step 3: Vapor Recovery and Compression
- Vapor Stream (S-3): Methanol-water vapor mixture exits column top at 65-85°C
- Mechanical Compression: Vapor is compressed to 0.8-1.2 MPa using energy-efficient compressors
- Temperature Rise: Compression increases vapor temperature to 120-140°C for heat recovery
Step 4: Heat Integration and Energy Recovery
- Primary Heat Exchanger: Compressed vapor preheats incoming methanol feed
- Reboiler Duty: Recovered heat provides 40-60% of column reboiler energy requirements
- Steam Generation: Excess heat generates low-pressure steam for other plant operations
Step 5: Water Separation and Methanol Recovery
- Condensate Separation: Compressed vapor is partially condensed to separate water-rich phase
- Methanol Recovery: Methanol-rich stream is returned to the process after purification
- Water Removal: Water stream (S-6) contains <5% methanol and is sent to wastewater treatment
Step 6: Product Recovery and Quality Control
- Bottom Product (S-5): High-concentration alkoxide solution (28-32 wt%) is withdrawn continuously
- Product Cooling: Product is cooled to 25-35°C in heat exchangers
- Quality Specifications: Final product meets NM 30 (30% sodium methoxide) or KM 32 (32% potassium methoxide) standards
Operating Conditions:
- Temperature Profile: 60-80°C (feed) → 120-175°C (reaction zone) → 25-35°C (product)
- Pressure: 0.3-2.5 MPa throughout the system
- Reflux Ratio: 2-5:1 for optimal water separation efficiency
This Evonik patent US11746075B2 describes a completely different process configuration than my previous single-column diagram. The patent specifically covers simultaneous production of both sodium AND potassium alkoxides using multiple columns.
Simultaneous production of sodium and potassium alcoholates
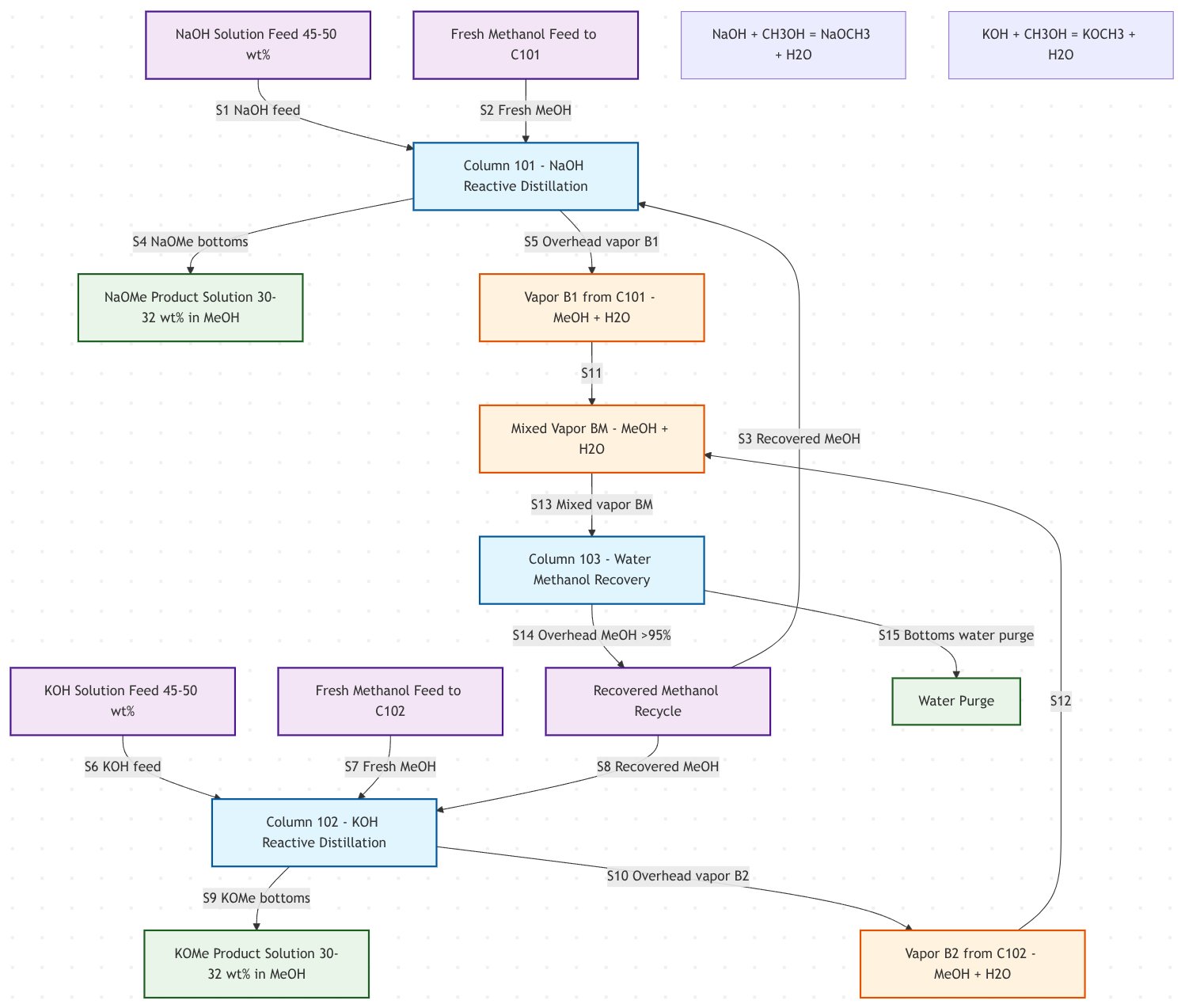
Evonik has developed a special process configuration for the simultaneous production of sodium and potassium alcoholates (US patent 11746075B2). Here are the key process configurations Evonik has developed (Fig. 2):
Two Separate Reaction Columns + Recovery Column:
- Column 101: NaOH + methanol → sodium methoxide
- Column 102: KOH + methanol → potassium methoxide
- Column 103: Combined vapor recovery column for methanol separation
More Advanced Configurations
- Dividing wall columns for simultaneous but spatially separated reactions
- Integrated rectification sections for methanol recovery
- Side columns for enhanced separation efficiency
Figure 2 - Evonik's simultaneous production of sodium and potassium methylates via the alkali hydroxide-methanol route (Appendix 2)
Process Efficiency
Conversion and Yield Performance:
- Hydroxide Conversion: >98% conversion of NaOH/KOH achieved through continuous water removal
- Alkoxide Yield: >95% yield based on hydroxide feed, with minimal side reactions
- Product Purity: Consistently achieves 28-32 wt% alkoxide content with <0.5% water and <0.5% free hydroxide
Selectivity Characteristics:
- Primary Reaction Selectivity: >99% selectivity to desired alkoxide products
- Side Reactions: Minimal ether formation (<0.1%) due to controlled temperature profile
- Impurity Control: Advanced process control maintains consistent product specifications
Energy Efficiency Metrics:
- Heat Recovery: 70-80% of reaction heat recovered through vapor recompression system
- Specific Energy Consumption: 1.2-1.8 GJ/tonne alkoxide product (industry benchmark: 2.5-3.5 GJ/tonne)
- Energy Reduction: 40-60% reduction in steam consumption compared to conventional processes, as claimed by the technology owner
Process Reliability:
- On-Stream Factor: >95% annual on-stream reliability through robust design
- Catalyst Life: Homogeneous process eliminates catalyst replacement requirements
- Maintenance Intervals: 18-24 months between major maintenance shutdowns
Commercial Experience
Global Production Network:
Evonik operates alkoxide technology across four continental locations,
- Germany (Niederkassel-Lülsdorf), now divested to LSF: Original technology development site with multiple production lines
- United States (Mobile, Alabama): Expanded capacity facility serving North American markets
- Argentina (Rosario): South American production hub with 50% capacity expansion completed
- Singapore (Jurong Island): Latest 100,000 tpy SMART plant representing state-of-the-art technology
Commercial Track Record:
- Production Capacity: Combined global capacity exceeds 400,000 tonnes per annum
- Market Position: Leading global supplier with approximately 25-30% market share
- Customer Base: Serves >200 customers across biodiesel, pharmaceutical, and specialty chemical industries
References
- Evonik. Company Fact Book 2024.
- J. Ruwwe et al. United States patent US7847133B2: Process for preparing alkali metal alkoxides. Priority date: May 27, 2008. Assignee: Evonik Operations GmbH.
- D. Roettger et al. United States patent US11746075B2: Method for the simultaneous production of sodium and potassium alcoholates. Priority date: Nov 30, 2020. Assignee: Evonik Operations GmbH.
- D; Roettger et al. European patent EP4074684B1: Method for the energy-efficient production of alkali metal ethanolates. Priority date: Apr 16, 2021. Assignee: Evonik Operations GmbH.
- M. Schröder et al. World patent WO2024120883A1: Improved process for preparing metal alkoxide compounds. Priority date: Nov 28, 2023. Application filed by Evonik Operations Gmbh.
- 赖明空 张辉. Chinese patent CN105884578A: Method for high temperature and high pressure production of sodium methoxide and device thereof. Priority date: Apr 21, 2016. Assignee: Individual.
- Evonik. Product Information: Sodium Methylate Solution, 28%.
- Evonik. Potassium Methylate Solution, 32%.
- Ministry of Trade and Industry, Singapore. Aug 27, 2025. Speech by Minister Grace Fu at the Evonik Singapore SMART (Sodium Methylate Advanced Recompression Technology) Grand Opening Ceremony.
- Evonik. Aug 27, 2025. Press Release: Evonik Celebrates Opening of World-Scale Alkoxides Plant in Singapore.
- Christoph Bauer. Sep 15, 2025. Climate-Neutral Production of Alkoxides. Elements - The Innovation Magazine by Evonik.
- Evonik. Mar 23, 2021. Evonik completes sodium methylate capacity expansion in Mobile, Alabama.
- ECHEMI. Jul 15, 2024. Evonik increases sodium methoxide production in Argentina.
- Advanced Biofuels USA. Dec 31, 2019. Find Out Why Sodium Methoxide Solution as a Biodiesel Catalyst Market is Thriving Worldwide by Top Key Players Like Evonik Industries AG,BASF SE
- Expert Market Research. Sodium Methoxide Market Size and Share Outlook: Forecast Trends and Growth Analysis Report (2025-2034).
Appendices
Perplexity A.I. generated Mermaid Chart codes,;
Appendix 1
View code and chart on Mermaidchart.com
flowchart LR
%% Feed Streams
A[Fresh Methanol Feed<br/>CH3OH]
B[NaOH Solution Feed<br/>45–50 wt%]
%% Equipment
E101[E-101 Heat Exchanger]
T101[T-101 Reactive Distillation Column<br/>120–175°C, 0.3–2.5 MPa]
C101{{C-101 Vapor Compressor<br/>ΔP=0.5–0.8 MPa}}
V101[V-101 Water Separator]
P101[P-101 Recycle Pump]
P102[P-102 Product Pump]
%% Products
WASTE[Waste Water]
PRODUCT[Sodium Methoxide Storage]
%% Streams and Details
T101 -- "Stream 3:<br/>Overhead Vapor<br/>MeOH + H2O" --> C101
C101 -- "Stream 4:<br/>Compressed Vapor" --> E101
E101 -- "Stream 5:<br/>Preheated MeOH to V101" --> V101
V101 -- "Stream 6:<br/>Water Purge<br/>(<5% MeOH, major H2O)" --> WASTE
V101 -- "Stream 7:<br/>Recycled MeOH<br/>(>95% MeOH)" --> P101
P101 -- "Stream 8:<br/>Recycle MeOH" --> T101
T101 -- "Stream 9:<br/>Bottoms Product<br/>30% NaOCH3 in MeOH<br/><0.5% H2O" --> P102
P102 -- "Stream 10:<br/>Final Product" --> PRODUCT
%% Reaction Box
RXNBOX[NaOH + CH3OH ⇌ NaOCH3 + H2O]
RXNBOX --- T101
%% Feeds
A --> E101
E101 --> T101
B --> T101
%% Styling
classDef equipment fill:#e1f5fe,stroke:#01579b,stroke-width:2px
classDef feed fill:#f3e5f5,stroke:#4a148c,stroke-width:2px
classDef product fill:#e8f5e8,stroke:#1b5e20,stroke-width:2px
class E101,T101,C101,V101,P101,P102 equipment
class A,B feed
class WASTE,PRODUCT product
Appendix 2
View code and chart on Mermaidchart.com
flowchart TD
%% Feed Streams
NaOH[NaOH Solution Feed 45-50 wt%]
KOH[KOH Solution Feed 45-50 wt%]
MeOH1[Fresh Methanol Feed to C101]
MeOH2[Fresh Methanol Feed to C102]
RecMeOH[Recovered Methanol Recycle]
%% Main Equipment
C101[Column 101 - NaOH Reactive Distillation]
C102[Column 102 - KOH Reactive Distillation]
C103[Column 103 - Water Methanol Recovery]
%% Products
NaOMe[NaOMe Product Solution 30-32 wt% in MeOH]
KOMe[KOMe Product Solution 30-32 wt% in MeOH]
Water[Water Purge]
%% Vapor Streams
V1[Vapor B1 from C101 - MeOH + H2O]
V2[Vapor B2 from C102 - MeOH + H2O]
VM[Mixed Vapor BM - MeOH + H2O]
%% Process Flow - Na column
NaOH -->|S1 NaOH feed| C101
MeOH1 -->|S2 Fresh MeOH| C101
RecMeOH -->|S3 Recovered MeOH| C101
C101 -->|S4 NaOMe bottoms| NaOMe
C101 -->|S5 Overhead vapor B1| V1
%% Process Flow - K column
KOH -->|S6 KOH feed| C102
MeOH2 -->|S7 Fresh MeOH| C102
RecMeOH -->|S8 Recovered MeOH| C102
C102 -->|S9 KOMe bottoms| KOMe
C102 -->|S10 Overhead vapor B2| V2
%% Mixing and recovery
V1 -->|S11| VM
V2 -->|S12| VM
VM -->|S13 Mixed vapor BM| C103
C103 -->|S14 Overhead MeOH >95%| RecMeOH
C103 -->|S15 Bottoms water purge| Water
%% Reaction Equations
RXN1[NaOH + CH3OH = NaOCH3 + H2O]
RXN2[KOH + CH3OH = KOCH3 + H2O]
%% Styling
classDef unit fill:#e1f5fe,stroke:#01579b,stroke-width:2px
classDef feed fill:#f3e5f5,stroke:#4a148c,stroke-width:2px
classDef prod fill:#e8f5e8,stroke:#1b5e20,stroke-width:2px
classDef vap fill:#fff3e0,stroke:#e65100,stroke-width:2px
class C101,C102,C103 unit
class NaOH,KOH,MeOH1,MeOH2,RecMeOH feed
class NaOMe,KOMe,Water prod
class V1,V2,VM vap