Technology Type
- Type
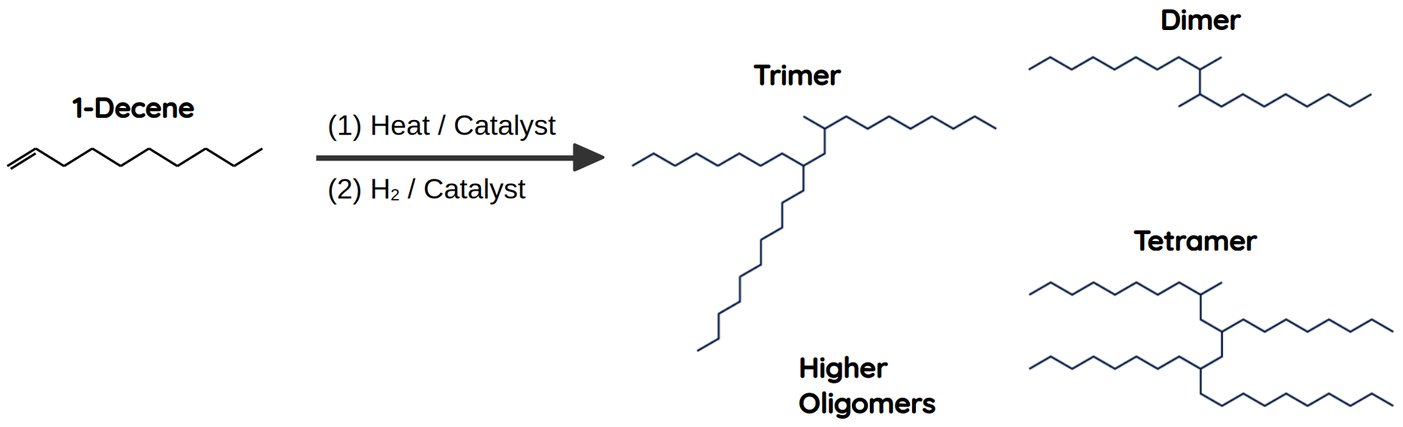
- Oligomerization of LAOs into PAOs
- Process
- Asphalt and Oil Processes
-

- #TT156
Description
Your insights will be shown here
| Technology | Owner | Entity |
|---|---|---|
|
|
Chevron Phillips | |

|
Neste |
Content provided by
| Transaction | Name | Date |
|---|---|---|
| Modified by |
|
10/2/2025 9:22 AM |
| Added | 9/9/2025 11:47 AM |