Combustion
Combustion, also known as burning, is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant, typically atmospheric oxygen[1],[2]. This process produces energy in the form of heat and light, commonly appearing as a flame[3].
Key Components
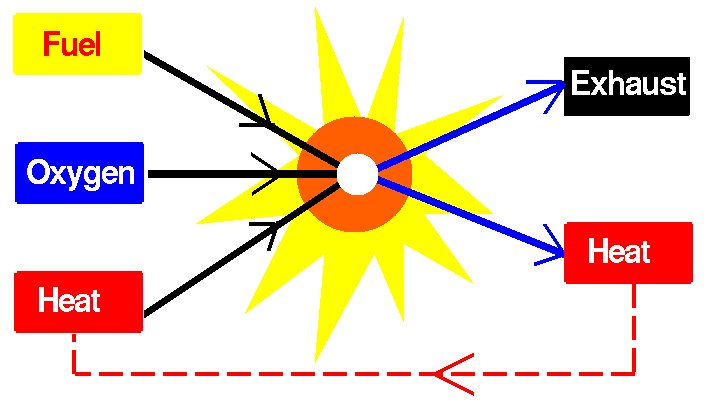
Three Essential Elements are required for combustion to occur[1] (Fig. 1):
- Fuel (the reductant)
- Oxygen (the oxidant)
- Heat (initial activation energy)
Figure 1 - Combustion is a chemical process that requires three elements[4]
Chemical Process
During combustion, the fuel reacts with oxygen to form new substances called exhaust products. For hydrocarbon fuels, the main products are typically carbon dioxide (CO2) and water (H2O)[1]. The heat generated during this process is measured as the calorific value or heating value, which represents the total energy released when a substance undergoes complete combustion[5]. For example, when hydrogen combusts with oxygen, it produces water vapor and releases 242 kJ/mol of heat[2].
Chemical Equations for Combustion
The simplest form of combustion is the burning of hydrogen. It combines two hydrogen molecules and one oxygen molecule to create water vapor:
2H2 + O2 → 2H2O + 286 kJ/mol of heat
Energy in the form of heat is produced because oxygen molecules are made up of two atoms with double bonds. When heat is added, the bonds break, releasing more energy.
The simplest hydrocarbon reactant is methane, CH4:
CH4 + 2O2 → CO2 + 2H2O + 890 kJ/mol of heat
The combustion of methane produces more heat per mole because the methane molecule has four single bonds between the carbon atom and each hydrogen atom.
Propane, which is C3H8, has two carbon-carbon bonds and eight hydrogen-carbon bonds:
C3H8 + 5O2 → 3CO2 + 4H2O + 2,220 Kj/mol of heat
Gasoline is a complex fuel, but the primary reductant is octane, where eight carbon atoms are bonded to 18 hydrogen atoms. That results in seven carbon-carbon bonds and 18 hydrogen-carbon bonds:
2C8H18 + 25O2 → 16CO2 + 18H2O + 5,483 kJ/mol of heat
A stoichiometric combustion reaction is theoretically ideal, in which the amount of fuel and oxygen are matched exactly, resulting in the most heat possible and maximum combustion efficiency[3].
Process Characteristics
The combustion reaction is exothermic, meaning it releases more heat energy than it consumes[3].
The process of heat generation through combustion is formally measured by its calorific value or heating value, which represents the amount of heat released during complete combustion of a specified amount of fuel[5].
Thermal Generation Systems
Thermal-generation systems are combustion-based heat systems that can be classified into two main types[6]:
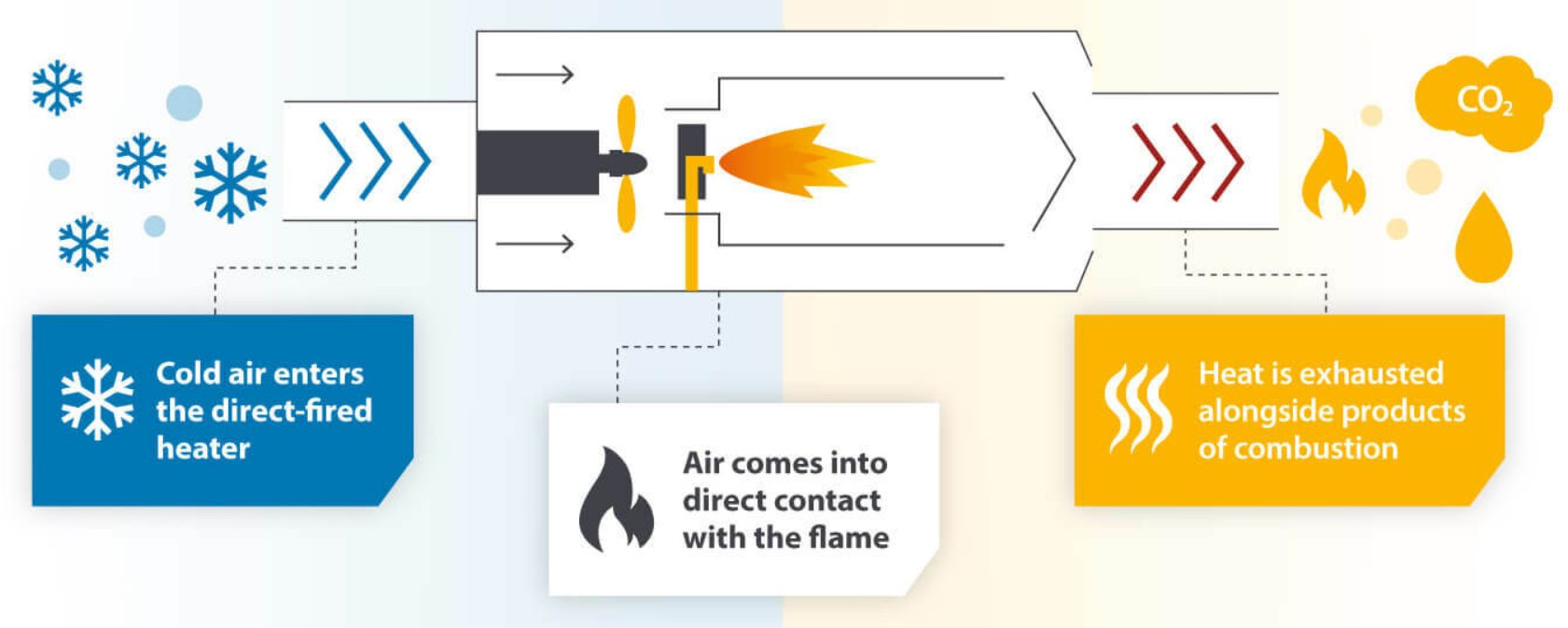
- Direct fuel-fired systems use burners with open flames that place hot combustion gases in direct contact with the material being heated. These systems are typically more efficient and offer better temperature control.
Figure 2 - Working principle of a direct-fired heater[7].
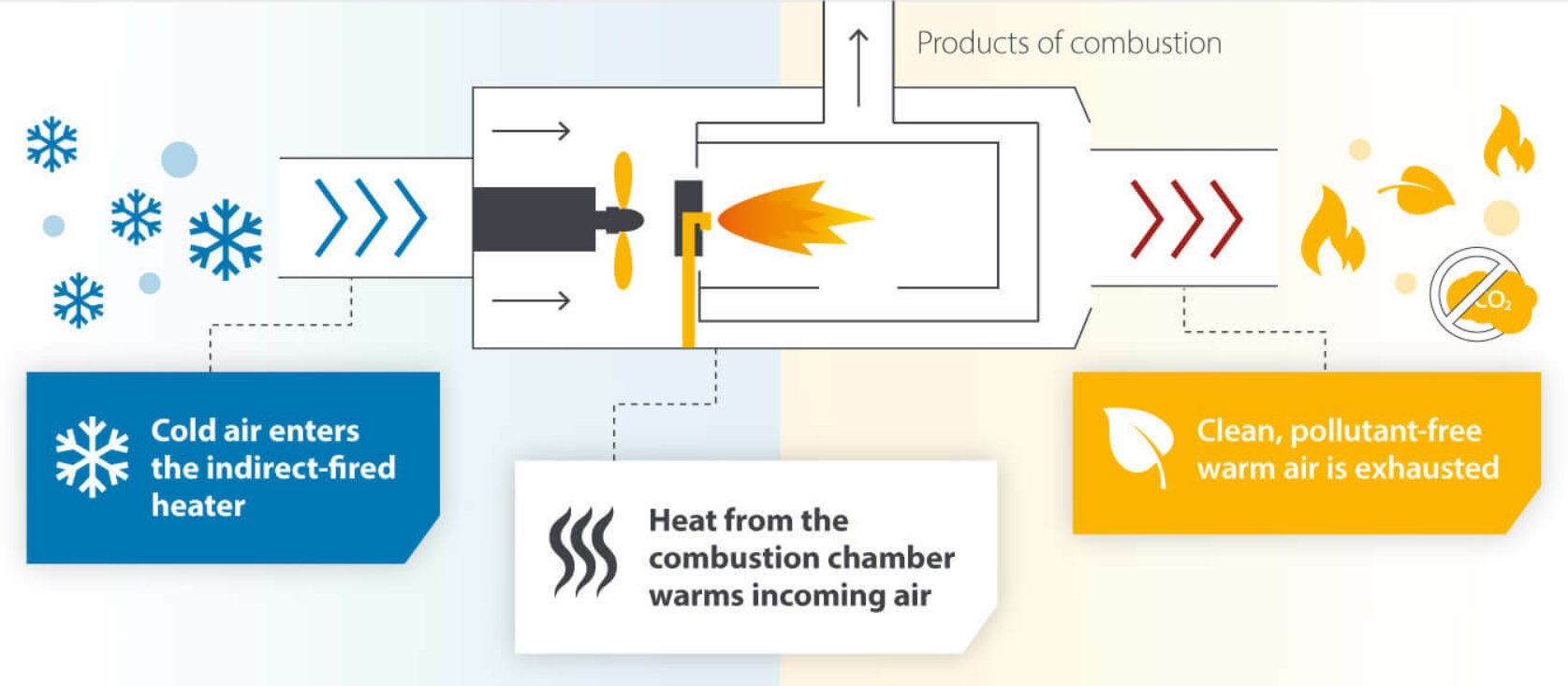
- Indirect fuel-fired systems contain flames in a sealed chamber, with heated gas flowing through tubes or panels that radiate heat to the material, keeping exhaust gases separate from the material being heated.
Figure 3 - Working principle of an indirect-fired heater[7].
Technical Classification
For pure heat generation applications, the systems can be referred to as[8]:
- Process heating systems;
- Thermal fluid heating systems;
- Combustion process heaters;
These systems can utilize various fuels including natural gas, oil, coal, biomass, or other combustible materials to generate thermal energy for industrial, commercial, or residential applications.
Performance Characteristics
Industrial combustion systems can achieve[9]:
- Operating times exceeding 8,400 hours annually;
- Availability rates above 96%;
- Retention times of 2 seconds above 850°C for waste processing;
- Optimized efficiency through reduced flue gas flow.
Emissions
In complete combustion, the emissions of combustion are water or, when carbon is present, water and carbon dioxide. However, most combustion involves other molecules, incomplete reactions, and secondary reactions that produce additional emissions. Any unwanted additional emissions are what we refer to as pollutants, and much of combustion science focuses on reducing these unwanted emissions.
In most cases, the nitrogen in air is inert and does not participate in combustion. However, oxygen can form bonds with nitrogen at high combustion temperatures to produce NOx. Also, when the amount of oxygen available is too low to react fully with the fuel, carbon monoxide can form instead of carbon dioxide. Volatile organic compounds can also form at low temperatures during combustion. These compounds with low boiling points easily react with other organic chemicals and produce unwanted pollutants[3].
References
- Dr. Stephanie R. Dillon, Florida State University, Chemistry for Liberal Studies, Forensic Academy, The Chemistry of Combustion.
- Wikipedia, Combustion.
- Ansys, Simulation Topics, What is Combustion.
- National Aeronautics And Space Administration - NASA, Combustion.
- Wikipedia, Heat of combustion.
- U.S. Department of Energy, Industrial Efficiency & Decarbonization Office, What Are the Different Kinds of Process Heating Systems?
- DANTHERMGROUP, Insights, 1st Oct 2024, Direct vs indirect-fired heaters: Advantages and disadvantages.
- Sigma Thermal, 25th Apr 2023, Industrial Process Heating Methods.
- HoSt Bioenergy, Technology, Combustion Technology.












