The Production of ethylbenzene emerges from a series of reactions, which begin with feeds of ethylene and benzene.
The simple reaction is listed:
C6H6 + C2H4 → C6H5C2H5
However, the ethylbenzene product reacts with ethylene to produce diethylbenzene:
C6H5C2H5 + C2H4 → C6H5(C2H5)2
Then, the diethylbenzene recycles with benzene to produce another yield of ethylbenzene:
C6H5(C2H5)2 + C6H6 → 2 C6H5C2H5
Finally, the trace amount of toluene in the benzene feed reacts with the ethylene feed to form two products, including more ethylbenzene:
C6H5CH3 + 2 C2H4 → C6H5C2H5 + C3H6
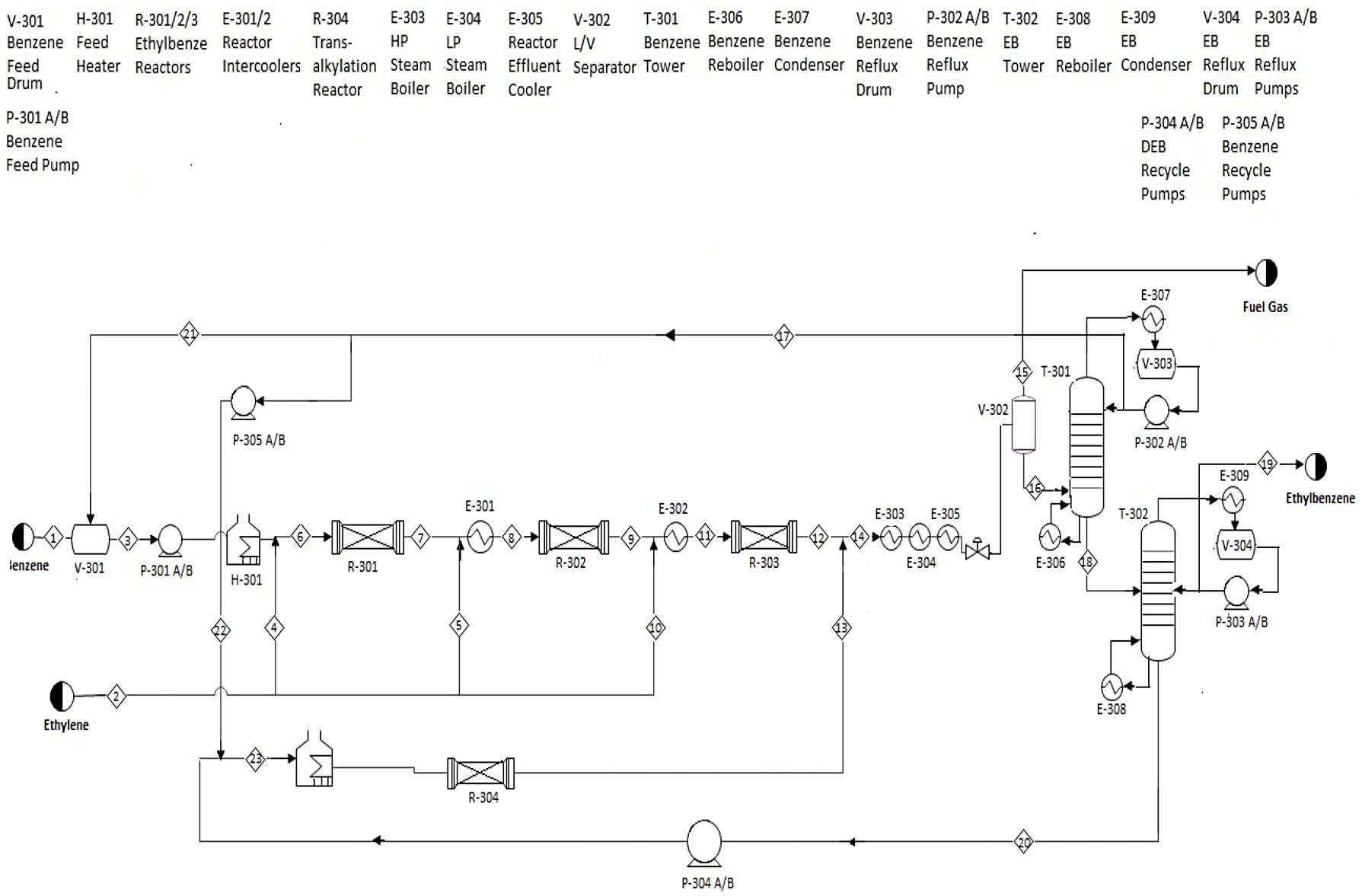
The benzene (97% benzene, 3% toluene) is prepared, using pumps, a vessel, and a fired heater, to react with three portions of the ethylene feed (93% ethylene, 7% ethane) in three reactors, each at 380°C. The product stream runs through three heat exchangers to cool to 80°C, after which the stream is pressurized (to 110 kilopascals) and a process vessel splits the product into fuel gas, which will be consumed in another fired heater, and a stream that feeds into the first distillation column. This column, T-301, separates most of the benzene and lighter hydrocarbons from a stream of ethylbenzene and diethylbenzene. The light stream is recycled with the benzene feed; the bottom stream feeds into a second column, T-302. This tower separates the ethylbenzene, at 99.8% purity and with <2 parts per million diethylbenzene, from a DEB-heavy bottoms stream. The bottoms stream gets sent through a pump, back through the fired heater, and another reactor with the same kinetics as the first three. This product joins the stream from R-303 before approaching the series of heat exchangers.
The process flow diagram for this process of production of ethylbenzene by alkylation of benzene with ethylene is presented in the following picture:
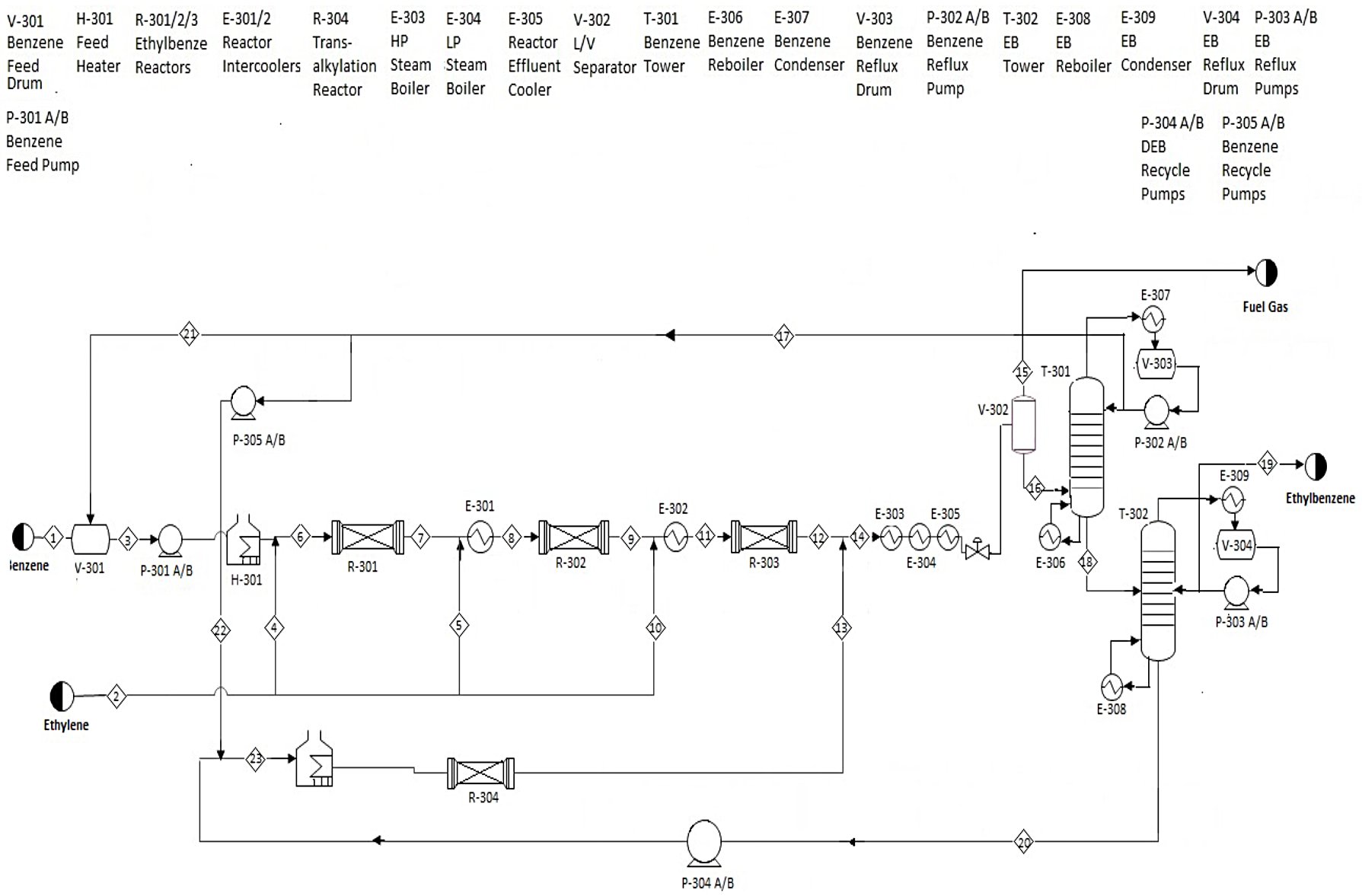
This diagram is based on the main reaction in the process, in which one mole of benzene reacts with one mole of ethylene to form one mole of ethylbenzene. The process has a fixed feed ratio (8:1) of benzene to ethylene, a purity of 99.8% in the ethylbenzene product stream, and a fixed annual production.
Source: Garrison Padgett Morgan, May 2015, Optimization of an ethylbenzene process, A thesis submitted to the faculty of The University of Mississippi in partial fulfillment of the requirements of the Sally McDonnell Barksdale Honors College, Oxford.