Historical Context
The Dimersol™ process represents one of the earliest and most successful applications of homogeneous catalysis in the refining industry, developed by the Institut Français du Pétrole (IFP) in the 1970s. The technology emerged as refineries sought efficient methods to upgrade light olefinic fractions, particularly propylene from fluid catalytic cracking (FCC) units, into higher-value products. The first industrial Dimersol™ unit began operation in 1977, marking a significant milestone in the commercial deployment of homogeneous organometallic catalysis for petroleum refining[1].
The development of the Dimersol-G™ process specifically addressed the needs of isolated refineries without petrochemical outlets for their propylene streams[2]. This technology provided an alternative to traditional alkylation processes, offering refiners flexibility to convert propylene into motor fuel components rather than leaving it as a lower-value byproduct[3]. The process was pioneered at Total Petroleum Inc.'s Alma, Michigan refinery, where it demonstrated superior performance in producing high-octane gasoline blending components[3].
Process Summary
The Dimersol-G™ process transforms light olefin fractions, primarily propylene from fluid catalytic cracking units, into high-quality gasoline components known as "Dimate". This gasoline blend exhibits exceptional properties including low boiling point and high octane characteristics, making it particularly valuable for gasoline pool enhancement[2]. The process operates through liquid-phase dimerization using a homogeneous nickel-based catalytic system that converts propylene into a mixture of hexene isomers with superior anti-knock properties[4].
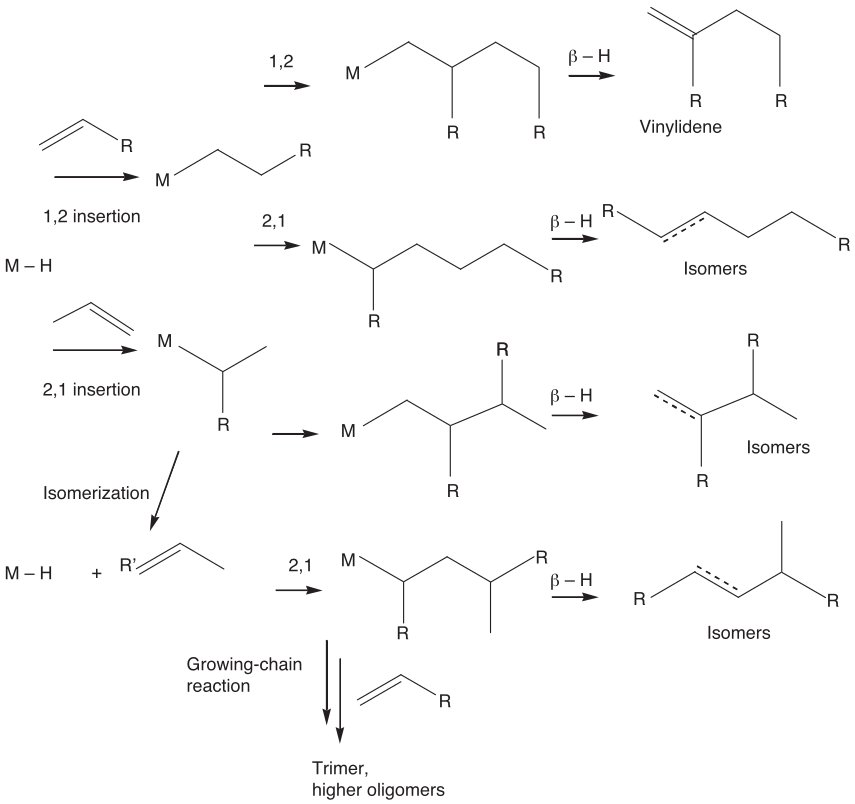
Figure 1 - Nonregioselective dimerization of propene catalyzed by cationic nickel complexes. General scheme for the formation of oligomers[4].
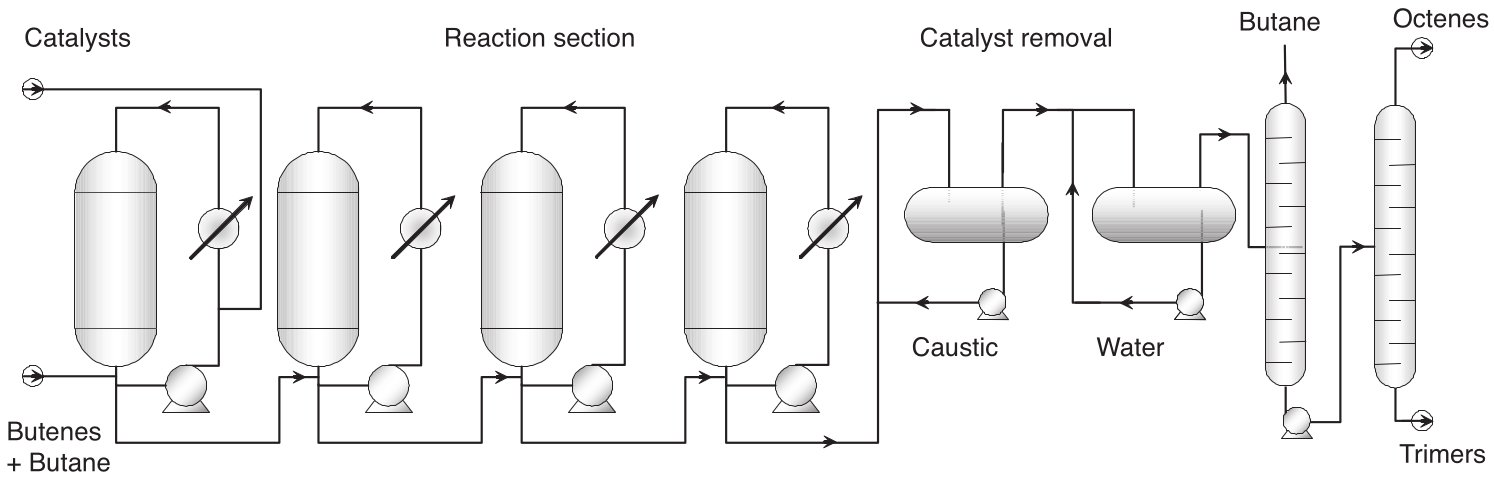
Detailed Process Description
Reactor System
- The Dimersol-G™ process employs a sophisticated liquid-phase reaction system operating under mild conditions with precise temperature and pressure control[4][5].
- The reaction section comprises multiple liquid-phase reactors arranged in cascade configuration, where propylene feed is continuously injected into the first reactor[4].
- The process utilizes a pump-around system with an external heat exchanger that uses water as cooling fluid to evacuate the exothermic heat of reaction, maintaining optimal reaction temperatures between 45°C and 55°C[5].
Catalyst
- The catalytic system consists of a two-component formulation where a nickel organic salt serves as the catalyst precursor and an ethylaluminum chloro derivative functions as the co-catalyst[4].
- These components are injected separately into the reaction loop under precise flow control, generating the active Ziegler nickel hydride catalyst in situ[4].
- The catalyst operates in a dilute hydrocarbon medium at relatively low pressure, typically around 22 effective bars, ensuring complete dissolution and optimal activity[4][5].
Reaction Control
- The reactor system maintains perfect mixing conditions while remaining completely filled with liquid phase throughout operation.
- Temperature control is critical, as the reaction kinetics are highly temperature-dependent, and the external heat exchange system provides precise thermal management.
- The catalyst deactivation is represented by first-order kinetics, and the system accounts for catalyst aging effects over time.
Product Handling
- Product separation occurs through a multi-stage process where the reactor effluent undergoes chemical neutralization to deactivate the catalyst.
- The neutralized catalyst residue is then removed from the hydrocarbon phase, followed by distillation to separate unreacted propylene from the desired hexene products.
Process Efficiency
- The Dimersol-G™ process efficiently converts propylene to valuable gasoline components, with the capability to process standard feedstock containing 70% propylene and 30% inert matter[5].
- The process achieves high conversion rates and high selectivity toward dimeric products while minimizing the formation of higher oligomers[5].
- At 50°C operating temperature, the isohexene distribution shows 22% n-hexenes, 72% 2-methylpentenes, and 6% 2,3-dimethylbutenes[4].
Process Economics
Typical case:
- Battery limits erected cost* (50,000 t/y) of a raffinate-2 C4 cut containing 75% n-butene: 6 million US$.
- Typical operating cost: $US 60 per metric tonne of octenes.
* 2018, Gulf Coast Basis excluding licensor fees and detailed engineering[6].
Commercial Experience
- As of 2018, 38 Dimersol™ units treating various olefinic C3 and C4 cuts have been licensed[6].
- As of 2009, 35 Dimersol plants, including both Dimersol-G and Dimersol-X variants, are operating worldwide, each plant produces between 20,000 and 90,000 tonnes per year of dimer products, contributing to a total annual production of 3.5 million tonnes[7].
Outlook
The Dimersol-G technology, which was widely adopted for converting propylene into high-octane gasoline components, likely lost traction in the U.S. market after the introduction of federal regulations limiting the olefin content in gasoline. The U.S. Environmental Protection Agency (EPA) enforced these restrictions as part of the Reformulated Gasoline (RFG) program beginning in 1995, with further tightening under the Tier 2 and Tier 3 standards in 2000 and 2017, respectively. These rules capped olefins at 10% by volume in summer gasoline blends to reduce ozone formation and improve air quality. As Dimersol-G’s main product is a highly olefinic gasoline blendstock, these regulatory changes significantly reduced its attractiveness for refiners focused on meeting compliance, leading to a decline in new installations and a shift toward technologies producing non-olefinic, regulatory-compliant fuels[7][8].
References
- Chauvin, Y. (2006). Olefin Metathesis: The Early Days (Nobel Lecture). Angewandte Chemie International Edition, 45(23), 3740–3747. doi:10.1002/anie.200601234
- Axens > Expertise > Oil Refining > Oligomerization > Homogeneous Oligomerization. (Accessed 22nd Jun 2025)
- Total Pet. Inc., Dimersol process for dimerizing propylene into a gasoline component scores high in commercial operation, Oil Gas J.; (United States) 78:17 (1980)
- A. Forestière et al., Oligomerization of Monoolefins by Homogeneous Catalysts, Oil & Gas Science and Technology – Rev. IFP, Vol. 64 (2009), No. 6, pp. 649-667
- A. Nica et al., DIMG92 : un simulateur de formation pour le procédé DIMERSOL, Rev. Inst. Fr. Pét., Vol. 48, No. 1, Jan-Feb 1993, pp. 53-60, DOI: 10.2516/ogst:1993004
- Axens, Dimersol-X Commercial Bulletin, Jun 2018. Downloaded from ref.[2]
- H. Olivier-Bourbigou et al., Ionic liquids and catalysis: Recent progress from knowledge to applications, Applied Catalysis A: General, Vol. 373, Issues 1–2, 2010, pp. 1-56, ISSN 0926-860X, DOI: 10.1016/j.apcata.2009.10.008.
- a. U.S. Environmental Protection Agency. "Reformulated Gasoline (RFG) Program." EPA RFG Overview and Regulations
b. U.S. Environmental Protection Agency. "Control of Air Pollution from Motor Vehicles: Tier 3 Motor Vehicle Emission and Fuel Standards." Federal Register, Vol. 79, No. 81, April 28, 2014.