Linear Alkylbenzene (LAB) is a key intermediate for manufacturing biodegradable detergents and surfactants, produced through the catalytic alkylation of benzene with linear mono-olefins derived from kerosene-separated n-paraffins that undergo dehydrogenation. LAB replaced earlier branched alkylbenzene (BAB) surfactants due to its superior biodegradability and cost-effectiveness.
Chemical Structure and Properties
Linear alkylbenzenes are a family of organic compounds with the formula C6H5-CnH(2n+1).
Typically, n lies between 10 and 16, although generally supplied as a tighter cut, such as C12-C15, C12-C13 and C10-C13, for detergent use.
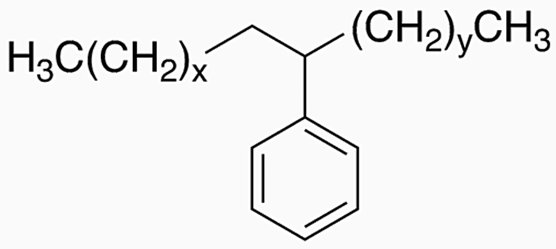
Linear alkylbenzene chemical structure
LAB is a clear, colorless to yellowish liquid with the following characteristics:
- Density: Approximately 0.86 g/cm³
- Boiling point: 290-310°C
- Flash point: Above 100°C
- Solubility: Insoluble in water but soluble in organic solvents such as alcohols and ethers
- Reactivity: Relatively stable under normal conditions but reacts with strong oxidizing agents
- Combustibility: Combustible and requires careful handling
Production of Linear Alkylbenzene
Linear alkylbenzenes are produced by 4 methods (technologies):
- Chlorination and monochloroparaffin method (early 1960s)
- Linear paraffins are chlorinated to form monochloroparaffin.
- An aluminum chloride catalyst is then used to alkylate benzene with monochloroparaffin.
- Chlorination and dechlorination to form olefins
- Linear paraffins are first chlorinated.
- They are then dechlorinated, forming olefins.
- Hydrochloric acid is used as a catalyst for the alkylation of benzene with the linear olefins.
- Dehydrogenation and Olefin Production through HF technology
- Linear paraffins undergo dehydrogenation to produce olefins.
- These olefins react with benzene in the presence of an HF acid catalyst to produce LAB.
- Unreacted paraffins are separated by distillation and returned to the beginning of the process.
- Dehydrogenation and Olefin Production through DETAL technology
- Normal paraffin in the range of C10-C13 are separated from Kerosene named heartcut.
- This heartcut is then hydrotreated to remove sulfur.
- Treated heartcut is then purified from aromatic compounds through MOLEX process.
- The treated heartcut is then goes through De-Hydrogenation process to produce olefins from normal paraffins.
- The olefins with Benzene are fed to alkylation reactor (DETAL) to produce LAB.
Primary Applications
Detergent Manufacturing: LAB is predominantly used as the raw material for producing Linear Alkylbenzene Sulfonic Acid (LABSA) by sulfonation with sulfuric acid or sulfur trioxide. LABSA is then neutralized with caustic soda to form sodium Linear Alkylbenzene Sulfonate (LAS), the most widely used surfactant in household and industrial detergents, including laundry powders, dishwashing liquids, and all-purpose cleaners.
Industrial Cleaning: LAB-based surfactants serve as powerful degreasers for machinery and equipment maintenance in industrial settings due to their excellent surfactant properties.
Specialty Applications: LAB functions as a base oil for specialty lubricants and functional fluids, offering superior low-temperature properties, oxidative and thermal stability, and elastomer compatibility. Additional uses include emulsifiers in pesticide formulations for agriculture, dyeing aids in textile manufacturing, and minor applications in ink production.
Environmental Advantages
LAB production with appropriate chain length (C10-C13) and improved linearity parameters contributes to higher biodegradation rates of the ultimate LAS detergent product. Modern LAB manufacturing generates no acidic discharge, eliminating the need for effluent treatment. These environmental characteristics, combined with cost-effectiveness, established LAB as the preferred replacement for non-biodegradable branched alkylbenzene surfactants in the 1960s.
References
- Shokri A, Karimi S.. A Review in Linear Alkylbenzene (LAB) Production Processes in the Petrochemical Industry (Feb 24, 2022). Russ J Appl Chem. 2021;94(11):1546–59. DOI: 10.1134/S1070427221110094. PMCID: PMC8867685
- Elchemy. Linear Alkyl Benzene Explained: Production, Properties, and Industrial Applications (Dec 22, 2025)
- Panorama Oil & Gas Sdn Bhd. Definition of LABSA
- GGT Petrochemical. Linear Alkyl Benzene (LAB)
- Green Gubre Group. LAB (Linear Alkylbenzene)
- Egyptian Petrochemicals Holding Company (ECHEM). Linear Alkyl Benzene
- Nanjing Chemical Material Corp.. Linear Alkyl Benzene (LAB)
- Indian Oil Corporation Ltd. Linear Alkyl Benzene (LAB)
- Equilex. Linear Alkyl Benzene (LAB)
- SOLTEX. Alkylate Fluids
- Wikipedia. Linear alkylbenzene




 CAS Number
CAS Number
 EC Number
EC Number
 ECHA InfoCard
ECHA InfoCard