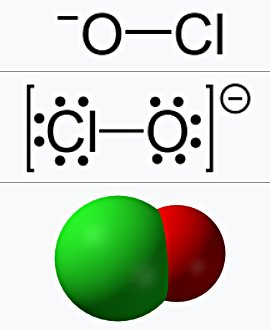
Hypochlorite is an oxyanion with the chemical formula ClO⁻, consisting of a chlorine atom bonded to an oxygen atom. It forms salts and esters known as hypochlorites, the most common of which are sodium hypochlorite (NaOCl) and calcium hypochlorite (Ca(OCl)2). These compounds are widely used as bleaching agents, disinfectants, and for water treatment.
Hypochlorite salts are typically handled in aqueous solution, as many are unstable in solid form—sodium hypochlorite, for example, is most often encountered as household bleach, a dilute solution used for cleaning and disinfection. Calcium hypochlorite is more stable and often supplied as a powder or pellets for pool sanitation and water treatment.
Chemically, hypochlorite is a strong oxidizing agent. It decomposes upon heating or acidification, releasing chlorine gas and forming chloride and oxygen or chlorate, depending on conditions. The conjugate acid of hypochlorite is hypochlorous acid (HClO).
In summary, hypochlorite refers to the ClO⁻ ion and its compounds, which are important industrial and household chemicals used primarily for their bleaching and disinfecting properties.