What is Chlorinated Polyethylene (CPE)?
Chlorinated Polyethylene (CPE) is a chlorinated polymer obtained through the chlorination of polyethylene. CPE is an inexpensive variation of polyethylene, where chlorine is substituted for some of the hydrogen atoms. CPE has a chlorine content from 34 to 44%.
History of CPE
CPE was discovered in the 1950s. Dr. James Barry of Dow Chemical noted that its properties make it effective in chemical and industrial applications, especially flexible tubing. The first chlorinated polymers were created by Dr. William Schockley, the co-inventor of the transistor. These materials were used in medical applications such as blood bags and dialysis machine parts to reduce the risk of bacterial infection exchanging bacteria with these materials would have on patients.
Characteristics and advantages of CPE
CPE possesses unique chemical structures and physical properties, such as:
- Excellent weather resistance and chemical resistance: CPE exhibits outstanding resistance to weathering and chemicals, protecting products from degradation caused by factors like UV radiation, ozone, acids, and alkalis, thus extending their service life.
- Superior heat resistance: CPE demonstrates high heat stability, allowing it to maintain its physical and mechanical properties under elevated temperatures. It is suitable for applications requiring heat resistance.
- Good flexibility and elongation: CPE offers excellent flexibility and elongation, making it an excellent elastomeric material. It retains its elasticity and deformation capability over a wide temperature range.
- Excellent flame retardancy: CPE has excellent flame retardant properties, slowing down the spread of flames and reducing the risk of fire. It finds wide application in flame retardant-demanding industries.
- Good processability: CPE exhibits good processability, enabling it to be blended with other plastic materials to achieve specific performance requirements and application needs.
Uses of CPE
Due to its superior performance and versatile characteristics, CPE is widely used in various fields, including:
- Construction and building materials: CPE is commonly used in thermal insulation materials, waterproofing membranes, pipe seals, flooring materials, etc., to provide weather resistance, chemical resistance, and heat resistance.
- Automotive industry: CPE finds extensive application in automotive seals, door trims, protective covers, etc., offering heat resistance, corrosion resistance, and flexibility.
- Cable and wire: CPE is utilized as insulation and jacketing material in cables and wires, providing high-temperature resistance, chemical resistance, and flame retardancy.
- Medical devices: CPE is widely employed in the medical device industry to manufacture medical gloves, tubing, seals, etc. It exhibits excellent chemical resistance and biocompatibility, meeting the requirements of the medical sector.
- Plastic products: CPE is used for the production of various plastic products such as plastic bags, containers, pipe fittings, etc. It enhances flexibility, weather resistance, and abrasion resistance in plastic products.
- Electronics and electrical equipment: CPE finds applications in the manufacturing of seals, insulation components, and protective parts for electronics and electrical equipment. It offers good electrical resistance and heat resistance, protecting electronic devices from external environmental impacts.
Due to its soft, rubbery texture, CPE is added to polyvinyl chloride to increase its impact and weather resistance. Furthermore, it is used for softening PVC foils, without risking plasticizer migration.
When selecting and applying CPE as a raw material, it is essential to consider specific needs and application requirements. Different types of CPE possess distinct characteristics and properties, allowing for the selection of the most suitable CPE product based on the product's requirements.
Production process of CPE
At present, the industrial chlorinated polyethylene (CPE) production plants that have been built in China generally use the aqueous phase production process, in which polyethylene
(PE) powder is dispersed in the aqueous phase medium, and chlorine gas is introduced to chlorinate the modified polyethylene to produce CPE products. The reaction process is as
follows:
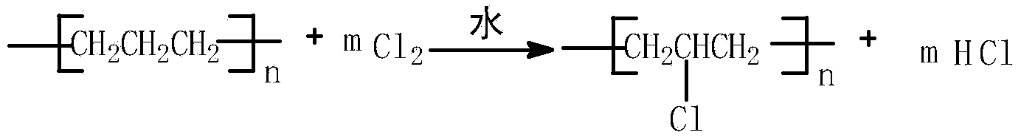
From the above reaction principle, it can be seen that in the production process of the aqueous phase method, hydrogen chloride products are generated, and hydrochloric acid is
formed in the aqueous phase system. The CPE generated is in a porous elastomeric polymer material, resulting in a large amount of hydrochloric acid adsorbed on the surface and inside the CPE material. The hydrochloric acid harms the CPE itself during processing and use, so the aqueous phase must have a deacidification kettle with a filter hood (deacidification process) to remove the mother liquor and to wash the material. To remove the adsorbed acid inside the CPE material, it is necessary to add a neutralization kettle in the aqueous process and add an alkaline neutralizer such as sodium hydroxide in the neutralization kettle further to remove the adsorbed acid inside the CPE material, resulting in a large amount of dilute hydrochloric acid wastewater with low concentration, which is costly to recycle. In actual industrial production, the wastewater is discharged by neutralization, a major environmental problem, and has large discharge wastewater.
Modus Operandi
In the chlorination reactor, water is added according to the required solid/liquid ratio, stirring is started, and the raw material polyethylene and various auxiliaries are added and
heated up according to the proportional measurement. When the temperature of the kettle liquid rises to a predetermined value, the liquid chlorine is vaporized by the gasifier and then passed to the kettle at a certain flow rate, and the chlorination reaction starts. Since the chlorination reaction is exothermic, cooling water is supplied to the reactor jacket immediately after the reaction starts to keep the reaction at a controlled temperature. When the accumulated chlorine flow reaches a certain value, the chlorine addition to the kettle is stopped. The material temperature is lowered, the pressure is reduced, and the air is blown into the kettle to remove unreacted chlorine gas. The reacted material is then sent to the de-acidification kettle to filter out the hydrochloric acid of about 8% by-product concentration. The de-acidified material is then washed. The dilute acid wastewater is discharged into a wastewater pond. After that, the CPE resin is washed several times, and the wastewater is discharged to the wastewater pond. After washing, water is added to the CPE to form a suspension and sent to the neutralization kettle, where the residual acid is neutralized with sodium hydroxide. After neutralization, the CPE resin is de-liquefied and washed by a centrifuge. The wet material is first dried in an air dryer and then dried in a boiling dryer. The dried CPE is ground and sieved by a grinder and sent to the silo for metering and packaging to obtain the finished CPE product.
Latest method for the preparation of CPE
The reaction of chlorination occurs in the presence of emulsifier, dispersant, initiator and chlorine gas, during which calcium oxide is added in batches of 5-7 parts by mass, and the
rate of calcium oxide addition is controlled at pH 7.0-8.5 to produce chlorinated polyethylene, polyethylene resin and calcium chloride aqueous solution; the resulting chlorinated polyethylene resin is centrifuged and dried to obtain the product chlorinated polyethylene; calcium chloride aqueous solution is concentrated and dried to obtain the by-product calcium chloride; the total amount of chlorine gas introduced is 11-22 parts by mass.
The main features are:
- said emulsifier is an anionic emulsifier and non-ionic emulsifier, the addition amount of 1-10% of the mass of high-density polyethylene.
- anionic emulsifier is an alkyl sulfonate emulsifier or alkyl alcohol sulfate emulsifier.
- dispersant is one or more hydroxy cellulose, polyolefin pyrrolidone, reactive silica, and polyvinyl alcohol, added at 5-8% of the mass of high-density polyethylene.
- initiator is an oil-soluble initiator; the addition amount is 1. 0-5. 0% of the mass of high-density polyethylene.
- the initiator is benzoyl peroxide, lauryl peroxide, or azo diisobutyronitrile.
- the reaction temperature of the chlorination reaction is 80-135°C, and the reaction time is 2-5 hours.