Main-Product
- Product
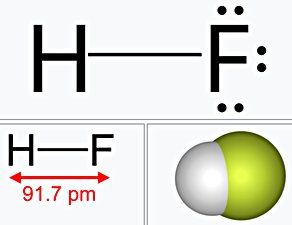
- Hydrogen Fluoride
- Segment
- Chemicals
- Main-Family
- Inorganics
- Sub-Family
- Mineral Acids
- Link
-

- #PS605
Description
Your insights will be shown here
Product Communicator
(*=Default)| Product | Title | Date | |
|---|---|---|---|

|
8/7/2024 | ||

|
2/25/2023 |
| Products (Quick Access) | Abbr. | Default |
|---|---|---|
| Hydrogen Fluoride | HF | |
| Hydrofluoric Acid | HF |
Settings
- Status
- A
- Unit of Measure
- Metric Ton
- Physical State
-
Liquid
Content provided by
| Transaction | Name | Date |
|---|---|---|
| Modified by |
|
8/4/2024 2:41 PM |
| Added | 2/25/2023 8:24 AM |