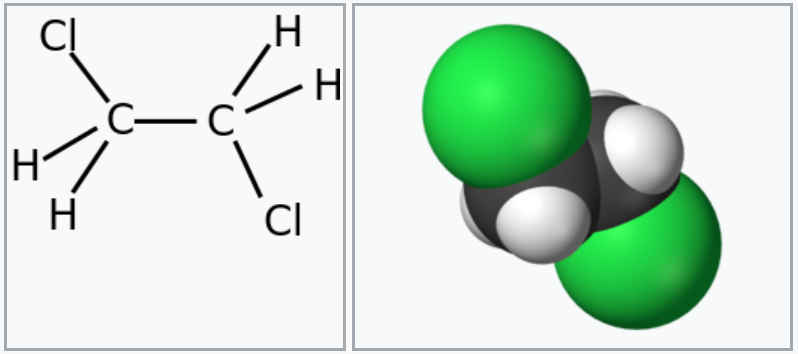
The chemical compound 1,2-Dichloroethane, commonly known as Ethylene Dichloride (EDC), is a chlorinated hydrocarbon with the chemical formula CH2Cl-CH2Cl. It is a colourless liquid with a Chloroform-like odour. The most common use of 1,2-Dichloroethane is in the production of Vinyl Chloride.
EDC Manufacturing
Figure 1 - Production of VCM by three different routes: acetylene hydrochlorination, ethylene direct chlorination/oxychlorination, and ethane oxychlorination[2].
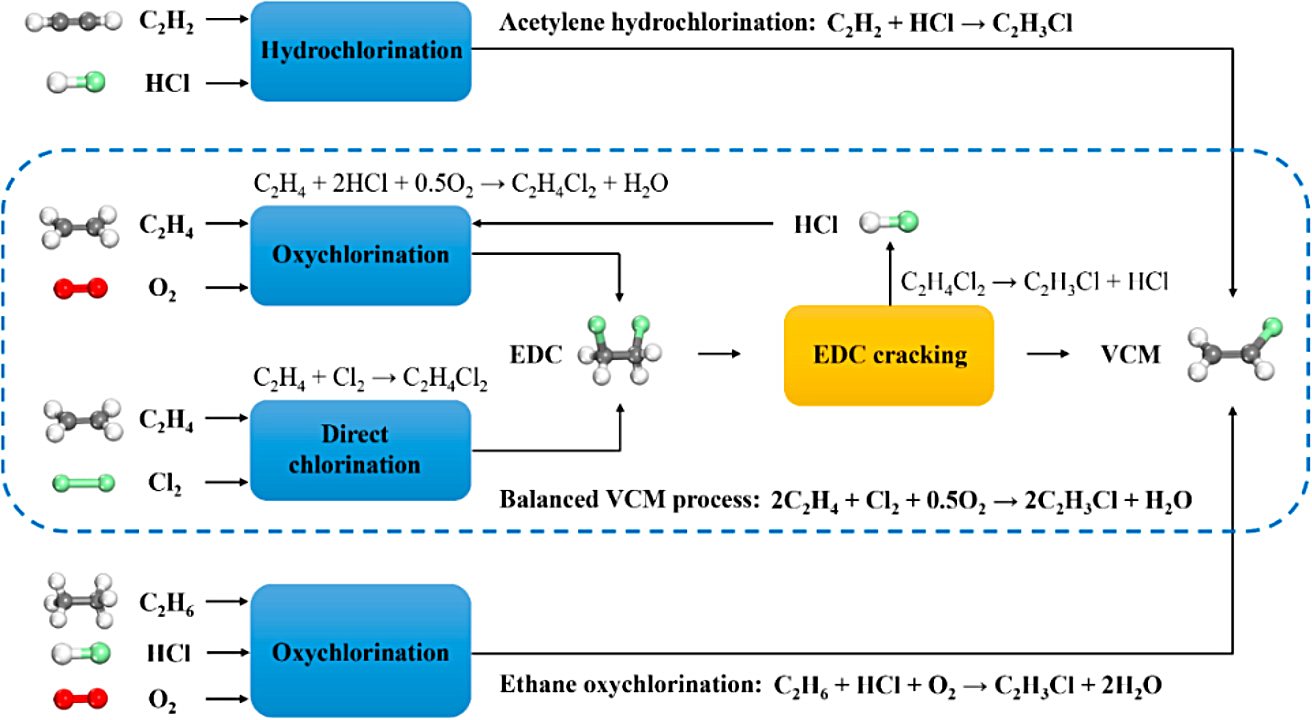
1,2-Dichloroethane production is primarily achieved through the Iron(III) Chloride-catalysed reaction of Ethylene and Chlorine (Ethylene Chlorination):
H2C=CH2 (g) + Cl2 (g) → ClC2H4Cl (l) (ΔH⊖r = −218 kJ/mol)
1,2-Dichloroethane is also generated by the Copper(II) Chloride-catalysed oxychlorination of ethylene:
H2C=CH2 + 2 HCl + 1/2 O2 → ClC2H4Cl + H2O
Utilization of EDC
Approximately 95% of the World's Production of 1,2-Dichloroethane is used in the production of Vinyl Chloride Monomer (VCM, Chloroethene) with Hydrogen Chloride as a byproduct. VCM is the precursor to Polyvinyl Chloride (PVC).
ClC2H4Cl → H2C=CHCl + HCl
The hydrogen chloride can be re-used in the production of more 1,2-Dichloroethane via the oxychlorination route described above.
References
- Wikipedia, 1,2-Dichloroethane.
-
Hongfei Ma, Yalan Wang, Yanying Qi, Kumar R. Rout, and De Chen, Critical Review of Catalysis for Ethylene Oxychlorination, ACS Catalysis 2020 10 (16), 9299-9319, DOI: 10.1021/acscatal.0c01698.