Electrolysis of Water[1]
Electrolysis of Water, also known as Electrochemical Water Splitting, is the Process of using Electricity to decompose Water into Oxygen and Hydrogen Gas by Electrolysis. Hydrogen Gas released in this way can be used as Hydrogen Fuel, or remixed with the Oxygen to create Oxyhydrogen Gas, which is used in Welding and other Applications.
Electrolysis of Water requires a minimum potential difference of 1.23 volts, though at that voltage external heat is required.
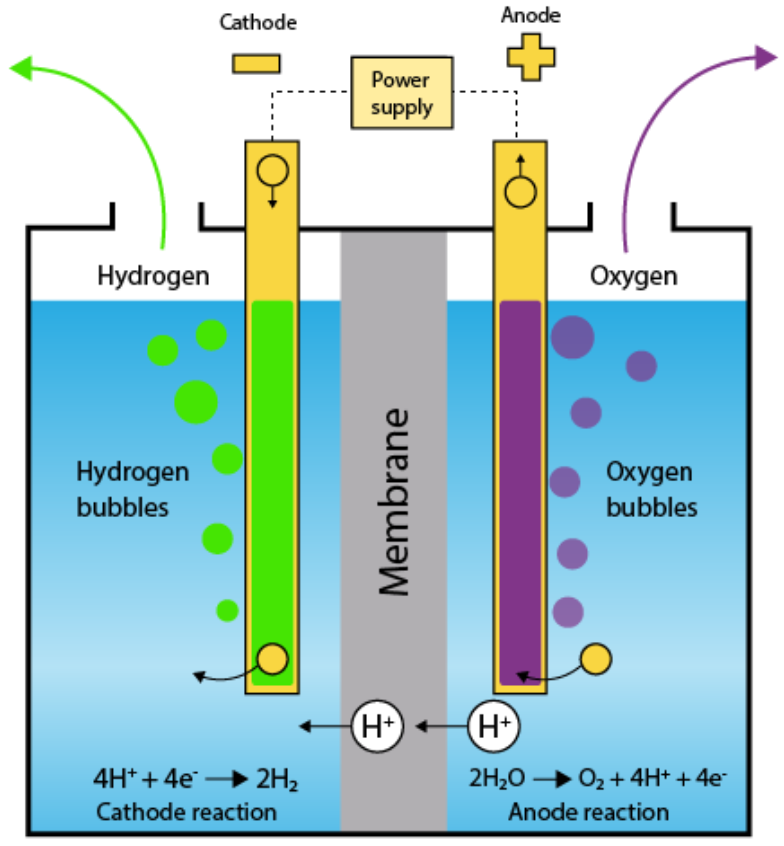
In Pure Water at the negatively charged Cathode, a Reduction Reaction takes place, with Electrons (e−) from the Cathode being given to Hydrogen Cations to form
Hydrogen Gas. The Half Reaction, balanced with Acid, is:
Reduction at Cathode:
2H+(aq) + 2e− → H2(g)
At the positively charged Anode, an Oxidation Reaction occurs, generating Oxygen Gas and giving Electrons to the Anode to complete the circuit:
Oxidation at Anode:
2 H2O(l) → O2(g) + 4 H+(aq) + 4e−
Combining Half Reaction Pairs yields the overall Decomposition of Water into Oxygen and Hydrogen:
Overall reaction:
2 H2O(l) → 2 H2(g) + O2(g)
Electrolysers[2]
The Electrolytic Cell used for the Electrolysis of Water is the Electrolyzer. Depending on the Transporter of the Electrolyte, Electrolyzer can be divided into three types;
-
Polymer Electrolyte Membrane (PEM) Electrolyzer
A Polymer such as Nafion separates the Electrodes and allows Hydrogen Ions formed by the Oxidation of water at the Anode to pass through it to the Cathode Compartment for discharge and form Hydrogen Gas.
-
Alkaline Electrolyzers
Dilute Aqueous Sodium (or Potassium) Hydroxide used in the Electrolysis provides a movement of Hydroxide Ions to the Anode to form Oxygen.
-
Solid Oxide Electrolyzer
Ceramic Oxide separates the Electrodes. At the Cathode, Water is reduced to Hydrogen and Oxide Ions. The Oxide Ions pass through the Ceramic Oxide to the Anode to become Oxygen Gas. This is used at high temperatures of 700 to 800°C to reduce the external voltage needed for Electrolysis.
References
1. Wikipedia, Electrolysis of Water
2. byjus.com, Water Electrolysis