Historical Background
The first commercially successful method for producing sulfuric acid was developed in 1746 by English physician and chemist John Roebuck (1718–1794). Roebuck's innovation, known as the lead chamber process, involved producing acid in large containers lined with lead, which were stronger, less expensive, and significantly larger than the previously used glass vessels. This breakthrough enabled the effective industrialization of sulfuric acid production, and the process remained the dominant manufacturing method for nearly two centuries, accounting for approximately 25% of global sulfuric acid production as late as 1946.
The contact process was invented around 1831 by Peregrine Phillips, a vinegar manufacturer from Bristol, England, who received British Patent No. 6096 for his innovation. Phillips described the instantaneous union of sulfur dioxide with atmospheric oxygen when the mixture passes over platinum heated to a strong yellow heat, with the resulting sulfur trioxide rapidly absorbed by water to form sulfuric acid. Although initially overlooked due to limited demand for concentrated sulfuric acid, the contact process gained prominence following the development of synthetic dyes and other industries requiring high-strength acid.
Lead Chamber Process
The lead chamber process involves three primary chemical reactions for converting sulfur to sulfuric acid:
S + O2 → SO2
2 SO2 + O2 → SO3
SO3 + H2O → H2SO4
In practice, sulfur dioxide is oxidized in the presence of nitrogen oxides (NOx), which act as catalysts. The process uses lead-lined chambers where a mixture of SO2, air, steam, and nitrogen oxides is introduced. The nitrogen dioxide oxidizes SO2 to SO3 (sulfur trioxide), which then reacts with steam to produce sulfuric acid at concentrations typically between 60–70%, known as "chamber acid". The nitrogen oxides are recovered and recycled, making their reuse an important economic consideration.
Althoug this method remains in limited use in some countries, the lead chamber process is now largely obsolete because it could not meet industrial demands for concentrated sulfuric acid and oleum production, had poor conversion efficiency (~30-50%), required complex heat management, suffered from NO2 recycling inefficiencies, and created excessive environmental emissions—all problems overcome by the contact process described in the next section.
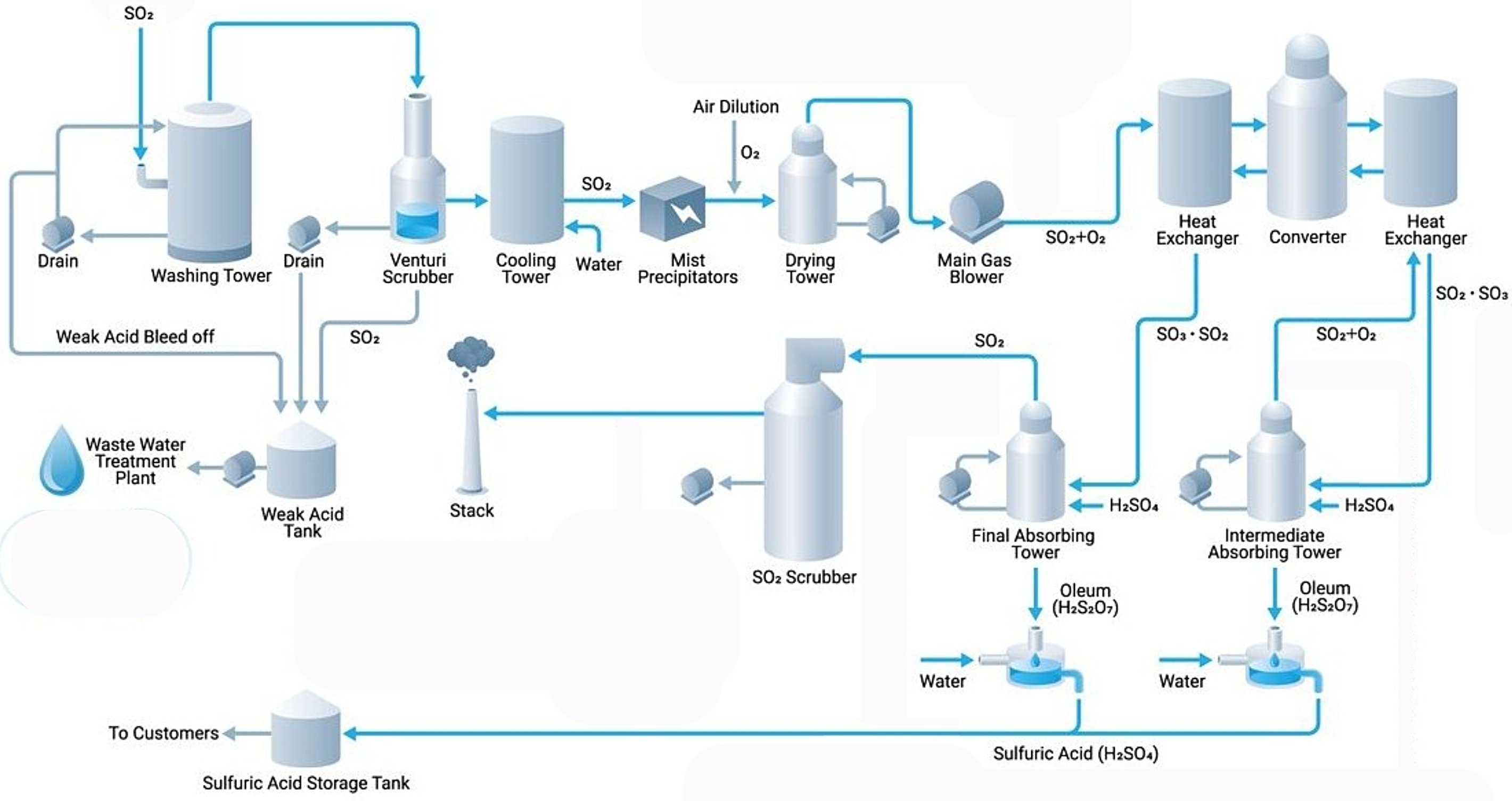
Contact Process
The modern contact process consists of four main stages:
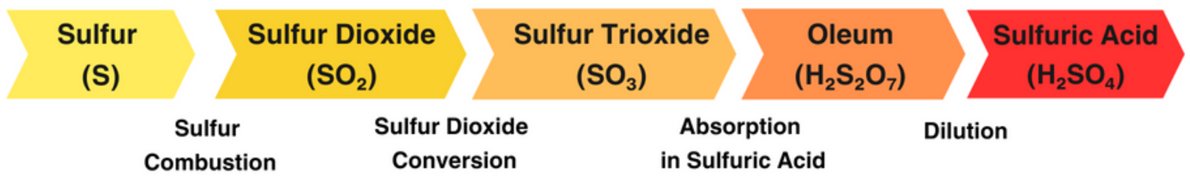
1. Sulfur Dioxide Generation (Combustion)
Sulfur dioxide (SO2) is produced by burning elemental sulfur in air or by roasting sulfide ores such as pyrite (FeS2):?
| S(s) + O2(g) → SO2(g) |
ΔHf @ 25°C = −70.94 kcal/mol |
2. Sulfur Dioxide Catalytic Oxidation into Sulfur Trioxide
The purified SO2 is oxidized to sulfur trioxide (SO3) over a solid catalyst in a multi-bed reactor:?
| 2 SO2 + O2 → 2 SO3 |
ΔH = −196 kJ/mol |
Modern plants use vanadium pentoxide (V2O5) as the catalyst, typically containing 4–9 wt% V2O5 as the active ingredient, supported on porous silica with alkali-metal sulfate promoters such as potassium sulfate or cesium sulfate. The catalyst is usually formed as cylindrical pellets or hollow rings to maximize surface area.
Process Conditions:
- Temperature: 420–550°C (optimally around 450°C)
- Pressure: Approximately 1–2 atmospheres (100–200 kPa)
- Excess oxygen: Used to drive equilibrium toward SO3 formation
The temperature represents a compromise between thermodynamic equilibrium (favoring lower temperatures for higher yields) and reaction kinetics (requiring higher temperatures for acceptable reaction rates). Catalyst bed inlet temperature optimization is crucial for maximizing conversion and minimizing SO2 emissions.?
3. Absorption of Sulfur Trioxide in Concentrated Sulfuric Acid
Direct reaction of SO3 with water is avoided due to its violent and explosive nature, which produces corrosive acid mist. Instead, SO3 is absorbed into concentrated sulfuric acid (98–99% H2SO4) to form oleum (H2S2O7, also called fuming sulfuric acid).
SO3 +H2SO4 → H2S2O7 (oleum)
4. Oleum Dilution in Water
Oleum is finally diluted with water to produce sulfuric acid at the desired concentration.
H2S2O7 +H2O → 2 H2SO4
Double Contact Double Absorption (DCDA) Process
Modern sulfuric acid plants predominantly use the double contact double absorption (DCDA) process, which achieves conversion efficiencies exceeding 99.5–99.8%. In this configuration, SO3 is removed from the gas stream after the third catalyst bed through an intermediate absorption tower, which shifts the equilibrium and increases the forward reaction rate for remaining SO2 conversion. The gas then passes through additional catalyst beds (typically 4–5 beds total) before final absorption. This process significantly reduces SO2 emissions compared to single contact single absorption (SCSA) processes, which typically achieve only 97–98% conversion.
Raw Materials
Sulfuric acid production utilizes three primary sulfur sources:
- Elemental sulfur from petroleum refining or natural gas processing
- Sulfide ores, particularly pyrite (FeS2), through roasting operations
- Waste sulfuric acid regeneration through thermal decomposition
Environmental Considerations
Modern sulfuric acid plants incorporate stringent emission controls to minimize SO2 releases. The DCDA process reduces unconverted SO2 emissions to levels below 200 mg/m³, with total conversion efficiencies above 99.7%. Additional control technologies include gas cleaning systems, Brink mist filters, and scrubbing methods that can convert residual SO2 back to sulfuric acid as a useful by-product rather than an atmospheric pollutant.
Key References
- The Iron Room (Jul 25, 2021). J. Roebuck.
- P. Phillips. (1831). British Patent No. 6096: Improvement in Manufacturing of Sulphuric Acid.
- W.G. Davenport et al. (2013). Sulfuric Acid Manufacture: Analysis, Control and Optimization (2nd ed.). Elsevier. ISBN 978-0-08-098220-5. DOI 10.1016/C2011-0-05490-X
- N.G. Ashar, K.R. Golwalkar (2013). A Practical Guide to the Manufacture of Sulfuric Acid, Oleums, and Sulfonating Agents. Springer International Publishing. ISBN 978-3-319-34810-0. DOI 10.1007/978-3-319-34811-7
- Chemical Engineering World (Sep 18, 2020). Sulphuric Acid Manufacturing Process
- Wikipedia (last edited: Sep 14, 2025). Lead Chamber Process
- Wikipedia (last edited: Mar 30, 2025). Contact process
- L.J. Friedman et al. (Document date: May 13, 2008). The History of the Contact Sulfuric Acid Process. Silo.tips
- BBC Bitesize (May 3, 2018). Sulfuric Acid - the Contact Process
- A. Shah Idil's HSC Notes (Document date: Jul 5, 2016). The Production of Sulfuric Acid
- F. Shokry et al. (Dec 2025). Process Simulation and Design of Sulfuric Acid Production via Double Contact Process. EgChem, Vol., 68 (12), 443-454. DOI 10.21608/ejchem.2025.362901.11346.
- W.G. Davenport et al. (Mar 2006). Sulphuric Acid Manufacture. Southern African Pyrometallurgy 2006, Edited by R.T. Jones, South African Institute of Mining and Metallurgy, Johannesburg.
- Sulphuric Acid on the Web (Mar 9, 2002). Technical Manual - Sulphur Burning - Sulphur Furnace
- M. Lashgari et al. ( May 25, 2023). SO2 pollutant conversion to sulfuric acid inside a stand-alone photoelectrochemical reactor: A novel, green, and safe strategy for H2SO4 photosynthesis. J. Ind. Eng. Chem. 121, 529–535 (2023). DOI 10.1016/j.jiec.2023.02.008
- J.-G. Wagenfeld et al. (Jul 15, 2019). Sustainable applications utilizing sulfur, a by-product from oil and gas industry: A state-of-the-art review. Waste Management, 95, 78-89. ISSN 0956-053X. DOI 10.1016/j.wasman.2019.06.002.
- R. Liu et al. (Dec 2021). Green and efficient comprehensive utilization of pyrite concentrate: A mineral phase reconstruction approach. Sep. Purif. Technol. 276, 119425 (2021). ISSN 1383-5866. DOI 10.1016/j.seppur.2021.119425
- American Chemical Society (Sep 14, 2025). Sulfur Trioxide
- T.K. Derry & Trevor I. Williams (Mar 24, 1993). A Short History of Technology: From the Earliest Times to A.D. 1900. ISBN 978-0486274720
- Smart Catalyst (accessed: Nov 8, 2025). Vanadium pentoxide catalyst