Process History
The catalytic oxidation of hydrogen chloride (HCl) to chlorine gas and water represents a mature industrial technology for recovering chlorine from waste HCl streams generated in chemical manufacturing processes, which is fundamentally based on the Deacon reaction, a form of reduction-oxidation (redox) reaction:
4 HCl + O2 → 2 Cl2 + 2 H2O
It has evolved through several generations of technological development, establishing itself as a commercially viable alternative to traditional chlorine production methods.
Early Development (1870s-1960s)
The foundation of HCl oxidation technology dates back to 1874 when Henry Deacon invented the original Deacon process for converting hydrogen chloride to chlorine using copper chloride catalysts at high temperatures (400-450°C). However, the early Deacon process faced significant limitations including low catalyst activity, severe corrosion issues, and thermodynamically limited conversion, preventing widespread commercial adoption.
The fundamental challenge of the original Deacon process lay in the equilibrium limitations at high temperatures and the rapid catalyst deactivation under the harsh operating conditions. These technical barriers limited early implementations and drove the search for improved catalyst systems and process configurations.
Commercial Breakthrough (1960s-1980s)
The 1960s marked a turning point with the development of three competing commercial processes: the Kel-Chlor process using nitrosylsulfuric acid catalysts, the Shell-Chlor process utilizing improved copper catalysts, and the MT-Chlor process employing chromium-based catalysts in a fluidized bed reactor. These second-generation technologies addressed many of the limitations of the original Deacon process through improved catalyst formulations and reactor designs.
The most successful of these early commercial processes began operation in 1988 and has maintained continuous commercial service for over 35 years, demonstrating the long-term viability of catalytic HCl oxidation. This operational history established confidence in the technology and paved the way for subsequent developments.
Modern Technology Evolution (1990s-Present)
The modern era of HCl oxidation has been characterized by multiple parallel technology developments focusing on different approaches to catalyst systems, reactor configurations, and process integration. Key advances include:
- Low-Temperature Catalysis: Development of highly active catalyst systems enabling operation at 250-350°C, significantly below historical temperatures while achieving superior conversion rates.
- Advanced Reactor Design: Implementation of multi-stage reactor systems with optimized heat management and catalyst utilization.
- Alternative Catalyst Systems: Innovation in non-precious metal catalysts that eliminate noble metal costs while maintaining competitive performance.
- Integrated Process Design: Development of fully integrated systems optimized for specific chemical manufacturing applications, particularly isocyanate production where HCl recovery provides maximum economic benefit.
Process Summary and Chemistry
Chemical Equation
The catalytic HCl oxidation process is fundamentally based on the Deacon reaction, which is an oxidation-reduction (redox) reaction forming chlorine and water as products:
4 HCl + O2 → 2 Cl2 + 2 H2O (ΔH = -59 kJ/mol)
This exothermic reaction achieves thermodynamic equilibrium that favors chlorine formation at moderate temperatures, making the process inherently energy-efficient compared to electrolytic alternatives.
Reaction Thermodynamics
The reaction thermodynamics favor high conversion at lower temperatures, with equilibrium conversion exceeding 95% at temperatures below 400°C when operating near stoichiometric oxygen-to-HCl ratios. The exothermic nature of the reaction (59 kJ/mol) provides significant heat release that can be recovered for steam generation or process heating.
The temperature dependence of the equilibrium creates an optimization challenge: higher temperatures increase reaction rates but decrease equilibrium conversion, while lower temperatures favor conversion but require more active catalysts to achieve acceptable reaction rates.
Reaction Mechanism
The catalytic oxidation proceeds via a Mars-van Krevelen type mechanism involving five key steps: hydrogen abstraction from HCl, chlorine atom recombination, hydroxyl recombination, water desorption, and dissociative oxygen adsorption. This mechanism explains the high selectivity to chlorine formation and the importance of catalyst composition in maintaining activity.
The mechanism requires catalysts capable of reversible oxidation-reduction while maintaining structural stability under the corrosive conditions of HCl and chlorine exposure. This requirement has driven the development of various catalyst formulations optimized for different operating conditions and performance priorities.
Detailed Process Description with Parameters and Conditions
The catalytic HCl oxidation process consists of several integrated unit operations designed to maximize conversion efficiency while managing the challenges of heat release and product separation.
Figure 1 - An example of HCl oxidation process flow[4]
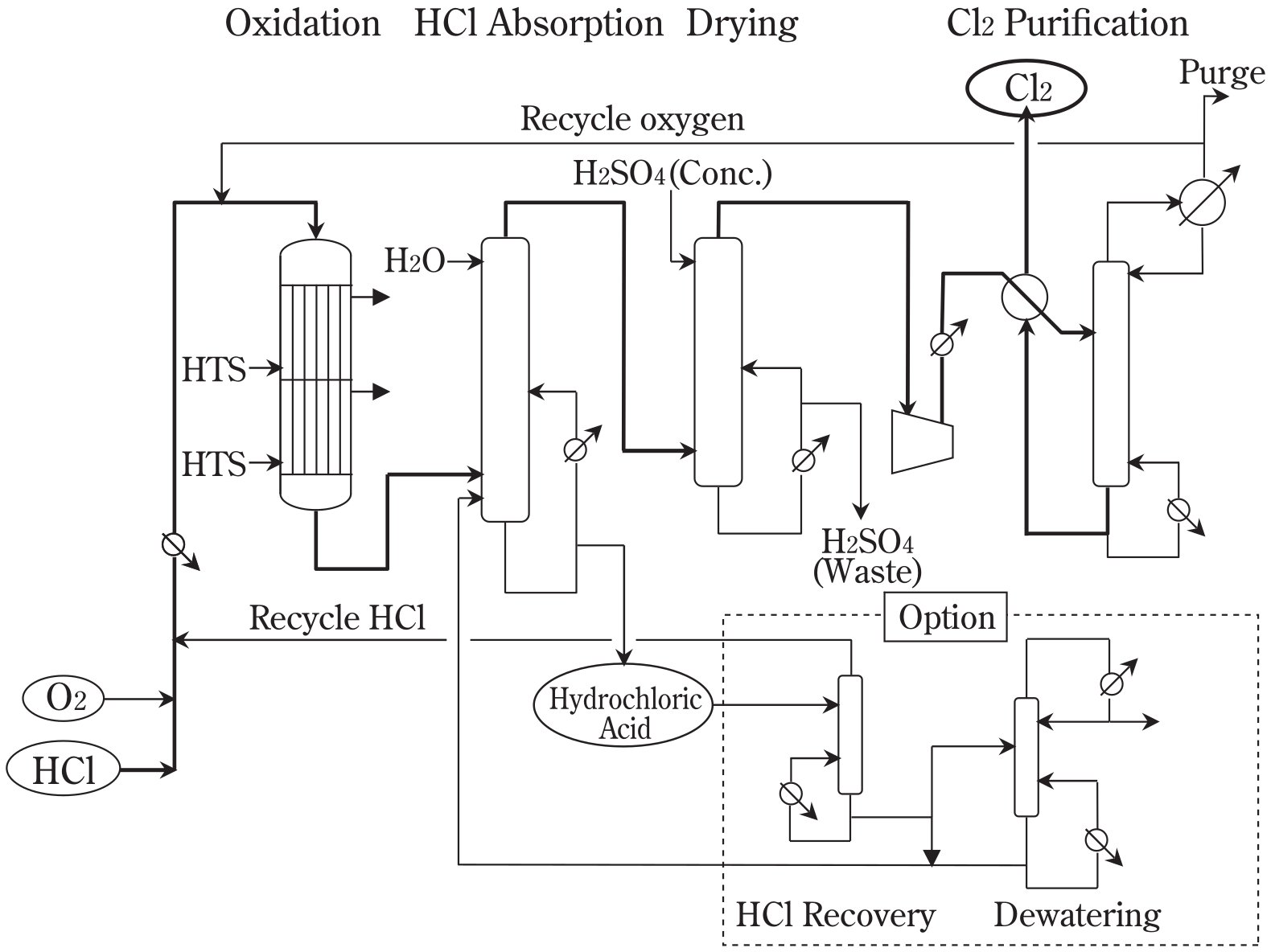
Feed Preparation and Conditioning
The process begins with feed gas preparation where hydrogen chloride gas and oxygen (typically supplied as air) are mixed in controlled stoichiometric ratios. The typical oxygen-to-HCl molar ratio ranges from 0.25 to 0.3, with the slight excess above the stoichiometric requirement of 0.25 ensuring complete HCl conversion while minimizing oxygen consumption.
- Feed Preheating: The mixed feed stream is preheated to reaction temperature using heat exchange with the hot reactor effluent. This autothermal design maximizes energy efficiency by utilizing the reaction heat for feed heating, reducing external utility requirements.
- Feed Purification: HCl feed streams often contain impurities from upstream chemical processes that can poison catalysts or reduce product quality. Feed conditioning may include removal of organic compounds, water content adjustment, and removal of catalyst poisons such as sulfur compounds.
Catalytic Reactor System
The heart of the process is the catalytic reactor system containing heterogeneous catalysts designed to promote the HCl oxidation reaction while maintaining long-term stability.
Reactor Configuration Options
- Fixed Bed Reactors: Utilize stationary catalyst beds with gas flow through the packed catalyst. This configuration provides precise temperature control and predictable residence time distribution, making it suitable for applications requiring high conversion and product quality.
- Fluidized Bed Reactors: Employ catalyst particles suspended in the gas flow, providing excellent heat transfer characteristics and the ability to continuously regenerate catalyst. This configuration is particularly suitable for applications with catalyst deactivation concerns or where isothermal operation is critical.
- Multi-Stage Reactors: Implement sequential reaction zones with different catalyst activities or operating conditions. This approach enables optimization of conversion while managing heat release and temperature control throughout the reactor system.
Operating Conditions
- Temperature Range: Modern catalytic systems operate between 250-450°C depending on catalyst type and conversion requirements. Lower temperatures favor equilibrium but require more active catalysts, while higher temperatures increase reaction rates but may reduce catalyst life.
- Pressure Conditions: Most commercial processes operate at atmospheric pressure to 3 bar gauge, eliminating the need for expensive high-pressure equipment while maintaining excellent reaction kinetics.
- Catalyst Loading: Reactor design must accommodate catalyst loading strategies that optimize conversion while preventing thermal runaway. This may include graded catalyst activity, dilution with inert materials, or staged catalyst addition.
Catalyst Systems
Catalyst Categories
Catalytic HCl oxidation employs various heterogeneous catalyst systems optimized for different operating conditions and performance requirements:
- Precious Metal Catalysts: Systems based on ruthenium, palladium, or platinum supported on oxide carriers. These catalysts typically offer the highest activity and selectivity but involve higher material costs.
- Transition Metal Oxide Catalysts: Formulations based on chromium, copper, iron, or vanadium oxides that provide good activity at moderate cost. These systems may require higher operating temperatures but offer excellent long-term stability.
- Non-Precious Metal Systems: Innovative catalyst formulations using proprietary combinations of base metals that eliminate noble metal costs while maintaining competitive performance.
Support Materials
Catalyst supports play a critical role in thermal stability, surface area, and catalyst-support interactions. Common support materials include:
- Titanium Dioxide (TiO2): Particularly in rutile form, provides excellent thermal stability and favorable electronic properties for precious metal catalysts.
- Silicon Dioxide (SiO2): Offers high surface area and chemical inertness, suitable for various catalyst formulations.
- Aluminum Oxide (Al2O3): Provides mechanical strength and thermal stability for high-temperature applications.
- Mixed Oxides: Specialized support compositions designed to optimize specific catalyst properties or operating conditions.
Product Separation and Purification
The reactor effluent contains chlorine gas, water vapor, unreacted HCl, and trace oxygen that must be separated to produce high-purity chlorine and recover unreacted feedstock.
Primary Separation
- Cooling and Condensation: Initial cooling removes most water vapor while maintaining chlorine in the gas phase. Temperature control is critical to prevent chlorine hydrate formation while achieving efficient water removal.
- HCl Recovery: Unreacted HCl is typically recovered through absorption and recycling, achieving overall HCl utilization rates exceeding 99%. This may involve scrubbing systems with subsequent HCl regeneration.
Chlorine Purification
- Final Product Quality: Modern catalytic processes achieve chlorine purity levels exceeding 99%, often surpassing the quality obtained from electrolytic processes. This high purity eliminates downstream purification requirements and enhances product value.
- Impurity Removal: Trace contaminants including oxygen, water, and organic compounds are removed through selective absorption, adsorption, or distillation depending on the specific impurities and quality requirements.
Heat Management
Heat Recovery and Utility Integration
The exothermic reaction requires sophisticated heat management to prevent temperature excursions that could damage catalysts or reduce conversion. Heat management strategies include:
- External Cooling: Reactor cooling systems using molten salt, thermal oil, or boiling water to remove reaction heat while maintaining optimal temperature profiles.
- Internal Heat Distribution: Catalyst bed design and gas flow patterns that promote uniform heat distribution and prevent hot spot formation.
- Heat Recovery Integration: Systems that capture reaction heat for steam generation, feed preheating, or integration with other plant utilities.
Steam Generation
Most commercial processes recover 60-90% of the reaction heat as medium-pressure steam, significantly improving overall process economics. Steam conditions typically range from 150-250°C and 5-20 bar depending on plant integration requirements.
Process Integration
Heat integration with other plant processes, particularly in integrated chemical facilities, can provide additional economic benefits through shared utilities and waste heat utilization.
Process Efficiency
Catalytic HCl oxidation processes demonstrate excellent efficiency characteristics that make them competitive with electrolytic alternatives.
Catalyst Performance
- Catalyst Life: Modern catalyst systems demonstrate operational life ranging from 1-5+ years depending on catalyst type, operating conditions, and feed quality. Longer catalyst life reduces operating costs and improves process economics.
- Activity Maintenance: Well-designed catalyst systems maintain stable activity throughout their operational life, with gradual deactivation patterns that enable predictive maintenance and replacement scheduling.
Conversion Performance
- Single-Pass Conversion: Modern catalytic systems achieve 70-98% single-pass conversion depending on operating conditions, catalyst system, and reactor design. Higher conversion rates generally require more active catalysts or more favorable operating conditions.
- Overall Conversion: With HCl recycle systems, overall conversion typically approaches 99%, maximizing chlorine recovery from available feedstock.
Selectivity Characteristics
- Chlorine Selectivity: Catalytic oxidation exhibits near-perfect selectivity to chlorine formation, with minimal formation of undesired byproducts. The heterogeneous catalytic mechanism ensures that virtually all converted HCl forms chlorine rather than other chlorinated compounds.
- Side Reaction Minimization: Properly designed catalyst systems minimize side reactions such as chlorine recombination or formation of higher chlorine oxides that could reduce product yield or quality.
Product Quality
- Chlorine Purity: Commercial catalytic processes typically produce chlorine with purity >99%, with leading processes achieving >99.7% purity. This high purity often exceeds that of electrolytically produced chlorine.
- Impurity Profiles: The catalytic process produces different impurity profiles compared to electrolytic chlorine, often with lower levels of certain contaminants that can be advantageous for specific applications.
Process Economics
The economic attractiveness of catalytic HCl oxidation depends on multiple factors including feedstock availability, energy costs, capital requirements, and product values within the context of the broader HCl processing market.
Capital Investment Requirements
- Reactor Systems: Catalytic oxidation requires moderate capital investment for reactor systems, heat exchangers, and separation equipment. The compact nature of catalytic reactors often results in lower equipment costs compared to electrolytic systems.
- Utility Infrastructure: Reduced electrical infrastructure requirements compared to electrolytic processes can significantly reduce capital costs, particularly for large-scale applications.
- Integration Costs: The ability to integrate with existing chemical facilities often reduces total capital requirements compared to standalone electrolytic installations.
Operating Cost Structure
- Raw Material Costs: The process requires HCl feedstock and oxygen (typically supplied as air), with minimal chemical consumption beyond catalyst replacement.
- Energy Consumption: Minimal electrical consumption compared to electrolytic processes (1,070-1,670 kWh/t for HCl electrolysis) provides significant operating cost advantages. Most energy input is thermal for feed heating and temperature maintenance.
- Catalyst Costs: Catalyst replacement represents a significant operating cost component, particularly for precious metal systems. However, long catalyst life (1-5+ years) and high activity help justify these costs.
- Utility Credits: Heat recovery as steam often provides utility credits that improve overall process economics.
Economic Comparison with Alternatives
- Versus HCl Electrolysis: Catalytic oxidation typically offers lower total production costs compared with HCl electrolysis due to reduced electricity consumption, despite catalyst costs. This economic advantage has driven the technology's growth within the HCl processing segment.
- Versus Brine Electrolysis: Catalytic HCl oxidation serves a different market segment (HCl recovery vs. primary chlorine production) and is not directly competitive with brine electrolysis (chlor-alkali process). The HCl-based market focuses on waste stream valorization and circular economy principles.
- Integration Benefits: In integrated chemical facilities, particularly those producing isocyanates or other HCl-generating processes, catalytic oxidation often provides superior economics through waste stream valorization and reduced disposal costs.
Technology Offering
The HCl oxidation technology market encompasses multiple technology providers, each offering different approaches to catalyst systems, reactor design, and process integration within the broader HCl processing market:
- Mitsui Chemicals operates the longest-running commercial HCl oxidation facility globally, with their MT-Chlor process maintaining continuous operation since 1988. Their chromium-based catalyst system in fluidized bed configuration has demonstrated exceptional long-term reliability with a 60,000 tons per year facility that represents one of the pioneering commercial implementations.
- Sumitomo Chemical and Technip Energies maintain extensive licensing activities for ruthenium-based catalyst technology, with over 10 licenses awarded and 7 operating commercial trains worldwide. Technip Energies holds exclusive global licensing rights since 2018, positioning this technology as a major competitor within the catalytic oxidation segment.
- Wanhua Chemical has developed innovative non-precious metal catalyst technology that eliminates noble metal costs while maintaining competitive performance. Their approach has been recognized internationally for sustainability innovation and supports multiple integrated facilities across China's growing chemical industry.
- Covestro (formerly Bayer MaterialScience) operates world-scale HCl oxidation facilities including installations with capacity exceeding 200,000 tons per year. Their internally developed multi-stage catalytic oxidation processes support global isocyanate production networks and demonstrate the scale potential of catalytic technologies.
References
- Wikipedia. Deacon process.
- Wikido. Deacon process.
- The Development of Improved Hydrogen Chloride Oxidation Process.
- H. Andon et al. Trends and Views in the Development of Technologies for Chlorine Production from Hydrogen Chloride. Sumitomo Kagaku, 2010-II, Report 1, 1-10.
- K. Iwanaga et l. The Development of Improved Hydrogen Chloride Oxidation Process. Sumitomo Kagaku, 2004-I, Report, 1-11.
- R. Weber et al. United States patent US20080029404A1: Processes for the production of chlorine from hydrogen chloride and oxygen. Priority date May 17, 2007. Application filed by Bayer MaterialScience AG.
- ECHEMI. Nov 20, 2021. Wanhua Chemical won a prize for MDI related technology.
- Covestro. Covestro’s new chlorine production plant Deacon II starts up in Shanghai.
- M. Ikeguchi et al. Hydrogen Halide Oxidation Process for Sustainable Halogen Recycling. Sumitomo Kagaku, 2021, Report 1, 1-13.
- N. López, et al. Mechanism of HCl oxidation (Deacon process) over RuO2. J. Catal. 2008, 255, 29-39.
- A.P. Seitsonen, H. Over. Dec 6, 2010. Oxidation of HCl over TiO2-Supported RuO2: A Density Functional Theory Study. J. Physic. Chem. C, Vol. 114, Issue 51, 22624-22629. SN 1932-7447. DOI: 10.1021/jp108603a.
- J. Liu et al. Ce-doped TiO2 supported RuO2 as efficient catalysts for the oxidation of HCl to Cl2. J. Environm. Sc., Vol. 149, Mar 2025, 234-241. ISSN 1001-0742, DOI 10.1016/j.jes.2024.01.017.
- MarketsandMarkets. Aug 2025. Report CH9485: Hydrochloric Acid Electrolysis Market.
- Chemical Processing. Nov 20, 2018. TechnipFMC Will License Sumitomo HCl Oxidation Technology.
- Technip Energies. Oct 2023. Technology Handbook.
