Process History
- The chlorohydrin process is the oldest industrial method for producing propylene oxide (PO), adapted from earlier ethylene oxide (EO) production technology.
- It was first commercialized in the 1950s and 1960s and became the dominant PO production route due to its technological maturity, flexibility, and relatively low capital investment requirements.
- Over time, environmental concerns regarding chlorinated by-products and wastewater led to the development of alternative processes (co-oxidation, hydrogen peroxide to PO), but the chlorohydrin method remains significant, especially in Asia and for integrated chlorine producers.
Process Overview and Chemistry
The chlorohydrin process involves two main steps:
-
Chlorohydrination: Propylene reacts with chlorine and water to form two propene chlorohydrin isomers and hydrochloric acid (HCl).
C3H6
|
+
|
Cl2
|
+
|
H2O
|
→
|
C3H7ClO
|
+
|
HCl
|
| propene |
|
chlorine |
|
water |
|
propene
chloro-
hydrin* |
|
hydro-
chloric
acid |
*mixture of 1-chloro-2-propanol and 2-chloro-1-propanol
Figure 1 - Propene chlorohydrin formation proceeds via a two-step reaction with formation of an intermediate propene chloronium complex (1), which subsequently reacts with water (2)
.jpeg)
|
(1)
|
.jpeg)
|
(2)
|
-
Dehydrochlorination (Saponification): The propene chlorohydrin isomers react with a base (typically calcium hydroxide or sodium hydroxide) to yield propylene oxide and salts (e.g., calcium chloride or sodium chloride)
C3H7ClO
|
+
|
Ca(OH)2
|
→
|
C3H6O
|
+
|
CaCl2
|
+
|
H2O
|
propene
chloro-
hydrin |
|
calcium hydroxyde |
|
propylene oxide |
|
calcium chloride |
|
water |
Figure 2 - The propene chlorohydrin isomers are dehydrochlorinated in the presence of a base, typically calcium hydroxide, into propylene oxide (3)
.jpeg)
|
(3)
|
Step-by-Step Process Description
1. Chlorohydrination
- Reactants: Propylene (polymer/chemical grade), chlorine (gaseous), water.
- Conditions:
- Temperature: 30–60°C
- Pressure: 2–6 atm
- pH: Maintained slightly acidic to neutral (pH 3–6)
- Operation:
- Propylene and chlorine are bubbled through water in a reactor (often a tower or loop reactor) to maximize gas-liquid contact.
- The reaction forms propylene chlorohydrin isomers and hydrochloric acid.
- Gaseous by-products are vented and scrubbed to remove chlorine and HCl.
2. Dehydrochlorination (Saponification)
- Reactants: Propylene chlorohydrin solution, calcium hydroxide (lime milk) or sodium hydroxide.
- Conditions:
- Temperature: 60–100°C
- Pressure: Atmospheric to slight overpressure
- Stoichiometry: Excess base is used to drive the reaction to completion.
- Operation:
- The chlorohydrin solution is mixed with the base in a saponifier.
- Propylene oxide is formed and separated from the aqueous brine containing calcium chloride (or sodium chloride).
- The brine is sent for treatment; PO is sent for purification.
3. Purification:
- PO is separated by distillation to achieve high purity (≥99.9%).
- Heavy ends and dichloropropane (DCP) by-products are removed and treated or used as fuel.
- Wastewater and brine are treated, often recycled to the chlor-alkali plant.
Typical Process Flow
| Step |
Main
Inputs |
Main
Outputs |
Typical
Conditions |
| Chloro-hydrination |
Propylene,
Cl2, H2O |
Chlorohydrin,
HCl |
30–60°C, 2–6 atm,
pH 3–6 |
| Dehydro-chlorination |
Chlorohydrin, Ca(OH)2 |
PO, CaCl2,
2O |
60–100°C, atm, excess base |
| Purification |
Crude PO,
rine |
Pure PO, DCP, waste brine |
Distillation,
drying |
Process Flow Diagram
A process flow diiagram for the PO hydrochlohydrin process is presented in Fig. 3.
Figure 3 - PO Hydrochlorhydrin process flow diagram
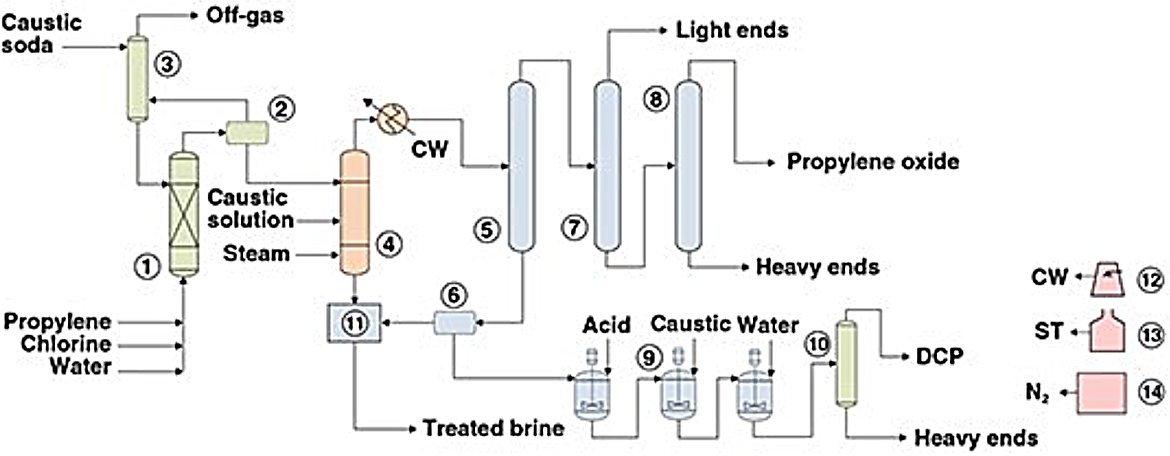
Legend: 1. Hypochlorination reactor, 2. PO decanter, 3. Caustic scrubber, 4. Saponifier, 5. Water removal column, 6. DCP decanter, 7. Light ends column, 8. Heavy ends column, 9. Acid/caustic/water washes, 10. DCP column, 11. Treatment unit, 12. Cooling tower, 13. Steam boiler, 14. Air separation unit, CW Cooling water, ST Steam, N2. Nitrogen
Technology Owners and Licensors
- Historical and Current Owners:
- Dow Chemical: Once the largest user and licensor of the chlorohydrin process.
- AGC (formerly Asahi Glass) and Tokuyama Corp.: Japanese producers using the technology.
- Chinese producers: Many older plants in China use the chlorohydrin process, though new construction is now restricted due to environmental regulations.
- Modern Licensing: Due to environmental restrictions, new chlorohydrin plants are rarely licensed outside China. The process is considered mature and is less frequently offered compared to newer, cleaner technologies.
Estimated PO Market Share by Process
- Historical Share: As of the mid-2000s, the chlorohydrin process accounted for about 50% of global PO production.
- Current Trends: The share has declined due to environmental pressures and the rise of alternative processes (hydrogen peroxide to PO, co-oxidation). Recent life cycle and market analyses estimate the chlorohydrin process now represents approximately 35-40% of global PO production.
- Regional Notes: The process remains significant in China (over one-third of capacity), but is being phased out in favor of cleaner technologies.
Advantages, Limitations, and Environmental Aspects
- Advantages:
- Mature, robust technology with flexible feedstock requirements.
- Lower capital investment compared to some co-oxidation routes.
- Limitations:
- Generates large amounts of chloride salts and wastewater.
- Environmental regulations increasingly restrict new installations outside integrated chlorine sites.
- Higher operating costs due to waste treatment and salt disposal.
- Environmental Impact: The process produces significant brine and chlorinated by-products, leading to stricter controls and plant closures in some regions.
References
- Aug 11, 2021 - Propylene oxide production method and process - Bloom Tech
- Propylene oxide - Wikipedia
- Junpei T SUJI et al., Development of New Propylene Oxide Process, R&D Report SUMITOMO KAGAKU, vol. 2006-I.
- 2021 - Propylene Oxide Production from Propylene and Chlorine - Report Propylene Oxide E11A Cost Analysis - United States - Intratec
- Hiroki Tanaka et al., Process for producing propylene chlorohydrin, United States Patent US7157609B2, Patent filed: May 21, 2003, Tokuyama Corp
- Mettler Toldeo - Apr 2017 - Application Note: pH Control in Propylene Oxide Production - Chemical Engineering Online
- T. Alexander Nijhuis et al., The Production of Propene Oxide: Catalytic Processes and Recent Developments, Ind. Eng. Chem. Res. 2006, 45, 10, 3447–3459, DOI: 10.1021/ie0513090
- 2017 - Process Data set: Propylene oxide production; technology mix; production mix, at plant; 100% active substance - ecoinvent
