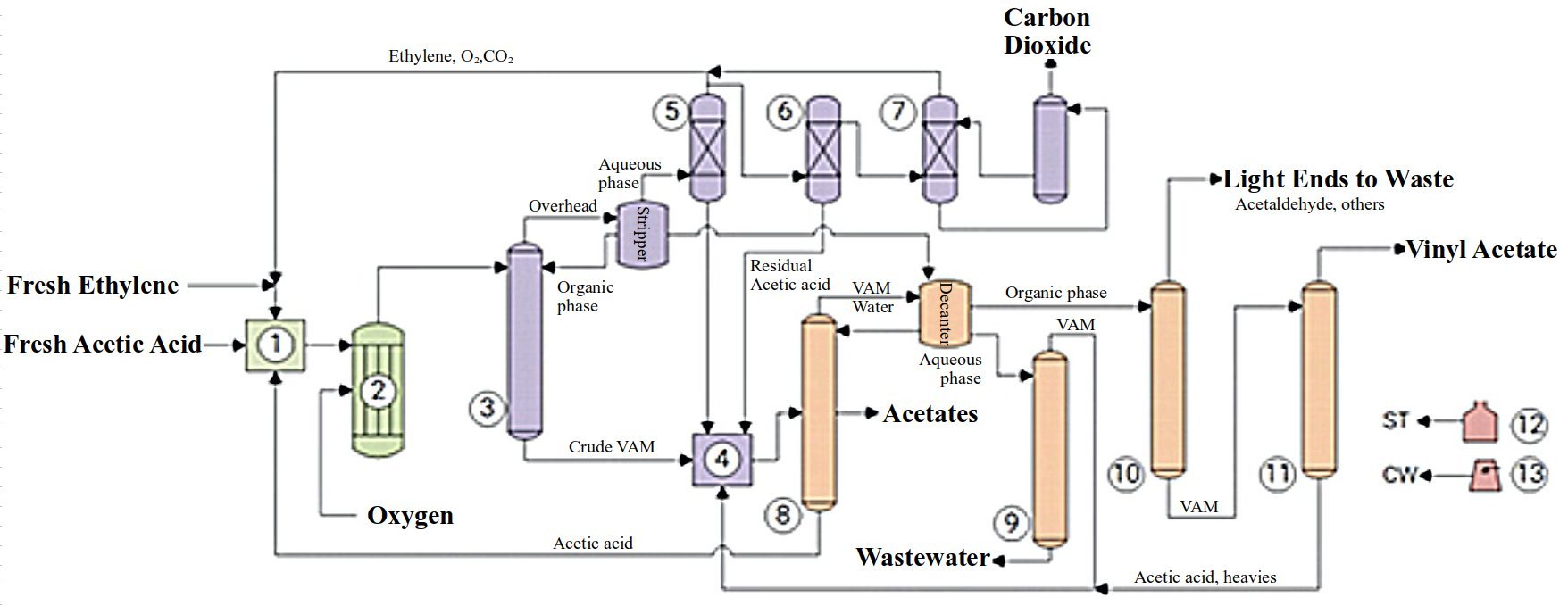
The production of Vinyl Acetate Monomer (VAM) from Acetic Acid and Ethylene (Picture) comprises three major Sections: Reaction; Separation; and Purification.
Reaction
Make-up and recycled Acetic Acid pass through a Vaporizer (1), along with fresh and recycled Ethylene. The Feed Stream, containing excess of Ethylene over Acetic Acid, is mixed with Oxygen, preheated and fed to Multi-Tube Reactors (2). The Reaction occurs over Palladium and Gold Catalysts. Heat is removed by Evaporative Cooling on the Reactor Shells. At the end, 8–10 wt.% of Ethylene and 15–35 wt.% of Acetic Acid are converted to Vinyl Acetate. Water, CO2 and small quantities of Ethyl Acetate, Ethylidene Diacetate and Glycol Acetates are the main Byproducts.[1]
Palladium and Palladium-based Catalysts are most commonly used to catalyze VAM Synthesis from Ethylene Acetoxylation with a selectivity up to 80%, according to the following Reaction[2]:
CH3COOH + C2H4 + 0.5 O2 → CH3COOCH=CH2 + H2O
Separation
The Reactor Effluent is cooled and fed to the Pre-Dehydration Column (3), where a Crude VAM Stream is withdrawn from the Bottom and stored. The Overhead Stream is separated into an Organic Phase, recycled to the Column, and an Aqueous Phase, directed to a Decanter Downstream. Uncondensed Gases are washed by Acetic Acid. The Solution formed is routed to the Crude VAM Tank (4), while Gases from the Scrubber (5) are recycled to the Reaction. Part of this Gas Mixture is washed with Water (6) to remove residual Acetic Acid, and also directed to the Crude VAM Tank (4). After Water Wash, Gases are directed to an Absorption Column (7), for CO2 removal by a Potassium Carbonate Solution.[1]
Purification
In the Azeotropic Column (8), a VAM-Water Mixture is distilled and fed to a Decanter, along with the Aqueous Phase separated in the Pre-Dehydration. Here, an Organic Phase containing VAM is separated and directed to the Light-Ends Column (10), while an Aqueous Phase is routed to the Wastewater Column (9), which separates residual VAM from Wastewater. Ethyl Acetate is withdrawn and discharged, and Acetic Acid is recycled to the Vaporizer (1). The Light Ends Column (10) strips off Acetaldehyde and other Volatile Compounds from the Crude VAM. Finally, Residual Acetic Acid and Heavy-Ends are removed in the pure VAM Column (11), yielding a Vinyl Acetate Product with 99.9 wt.% purity.[1]
Existing Technologies
VAM is commonly produced by the following Processes:
- CELANESE Ethylene-based VAM technology, formerly known as VAntage® and VAntagePlus™ and VAntage®2 VAM Technologies.
- INEOS Ethylene-based VAM Technology using a Fixed Bed Multi-Tubular Reactor, acquired from British Petroleum in 2008, a version of which the Fixed Bed Reactor is replaced with a Fluidised-Bed Reactor was branded Leap Technology.
- KBR Alliance with Showa Denko K K (SDK), Ethylene-based VAM Technology.
References
- 1st Nov 2021, Technology Profile: Production of Vinyl Acetate, CHEMICAL ENGINEERING
- Yanping Huang, Xiuqin Dong, Yingzhe Yu, Minhua Zhang, Kinetic Monte Carlo study of vinyl acetate synthesis from ethylene acetoxylation on Pd(100) and Pd/Au(100), Applied Surface Science, Volume 423, 2017, Pages 793-799, ISSN 0169-4332