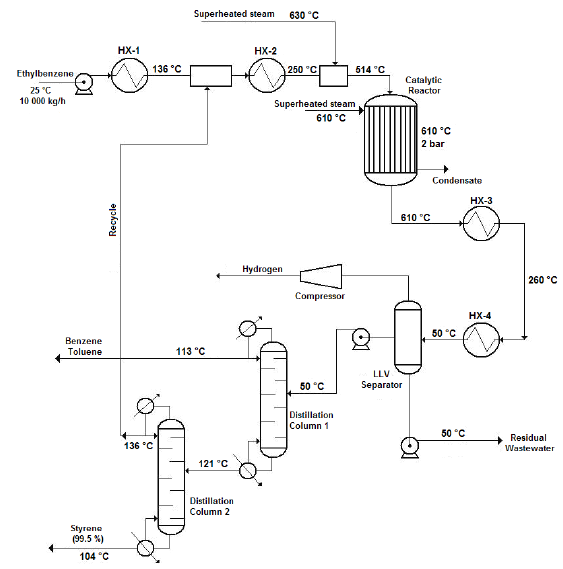
Catalytic ethylbenzene dehydrogenation process summary
Styrene is produced industrially through several production processes, the most important one being the catalytic dehydrogenation of ethylbenzene at high temperature (630 °C) using various metal oxides as catalysts, such as zinc, chromium or magnesium oxides coated on activated carbon, alumina or bauxite.
Catalytic dehydrogenation of ethylbenzene is composed of four stages:
- Ethylbenzene preheating, mixing and vaporization
- Dehydrogenation
- Cooling
- Separation/Purification.
The illustration shows a typical flow diagram of the styrene production process via catalytic dehydrogenation of ethyl-benzene.
Detailed process description
1. Ethyl-benzene pre-heating, mixing and vaporization
The production process starts when a liquid ethyl-benzene stream is pre-heated from ambient temperature (25 °C) to a temperature of about 136 °C using a shell and tube heat exchanger. The pre-heated stream obtained is then mixed with a recycle stream containing mostly ethyl-benzene and water, and some traces of styrene and toluene, coming from the top of the Distillation Column No. 2 (Styrene Column) in a cylindrical, pressurized vessel (Streams Mixer), operating under isobaric conditions. The exit stream leaving the Stream Mixer is completely vaporized in another shell and tube heat exchanger, until it reaches a temperature of about 250 °C. The vapors obtained are then sent to another pressurized vessel operating also under isobaric conditions (Steam Mixer), at which superheated steam is injected in order to increase the temperature of the vapor mixture to that of the reaction condition (600 °C). The amount of superheated steam to consume in the Steam Mixer should be enough to obtain a gaseous stream with a final water/ethyl-benzene molar ratio of approximately 14:1, prior to being fed to the Conversion Reactor.
2. Catalytic dehydrogenation reaction
The ethyl-benzene catalytic dehydrogenation takes place in a vertical cylindrical shell and tube reactor (Catalytic Reactor), which operates isothermally. The reacting gaseous mixture flows inside the tubes over the catalyst bed, while superheated steam is injected in the reactor’s shell to maintain the reaction temperature in the requested range (580 - 610 °C). The reaction temperature must not be increased to a value higher than 610 °C since thermal decomposition of both ethyl-benzene and styrene would occur. At the reactor’s exit a hot gaseous mixture is obtained, which has a styrene mass composition of about 93 % (on dry basis).
3. Cooling
The hot gaseous mixture coming from the Catalytic Reactor, which presents a temperature near 600 °C and a pressure of 3.5 bar, is pressurized to 6.0 bar using pressure regulating valves. Then, the mixture is cooled to 50 °C via two shell and tube heat exchangers (Coolers), which use cooling water as the heat exchanging agent. A two-phase (vapor-liquid) stream with approximately 50ºC temperature is obtained at the exit of the second cooler, which is then sent to the Separation/Purification area.
4. Separation/Purification
The styrene present at the exit stream of the Conversion Reactor must be separated and purified from the rest of the other chemicals contained on it (water, toluene, etc.). To accomplish this, the two-phase stream obtained at the exit of the coolers is sent to a three-phase separator (Liquid-Liquid-Vapor Separator or LLV Separator), which operates at 5 bar and 50 °C. A gaseous mixture primarily composed by hydrogen, methane and ethylene (as well as minor amounts of water and styrene) is obtained at the top of the LLV Separator (light gaseous stream), while a liquid stream containing water and certain traces of styrene, benzene and ethylene is obtained at the bottom of this equipment (wastewater stream). The middle stream (useful stream) contains mainly styrene, as well as minor amounts of the other chemicals, and is sent to the Distillation Column No. 1 (Benzene-Toluene Column), in order to recover both the benzene and toluene contained on it, and also for styrene concentration.
Both the benzene and toluene are obtained at the top of the Distillation Column No. 1 while a styrene-rich stream is obtained at the column’s bottom, which is sent to the Distillation Column No. 2 (Styrene Column) to carry out the final purification step. At the Styrene Column, the styrene is obtained at the bottom stream with a purity of about 99.5 % while the stream obtained at the top of this column (composed mostly by ethylbenzene, benzene and toluene) is recycled back to the process (Recycle Stream) and mixed with the pure ethyl-benzene stream which had previously been pre-heated at the first shell and tube heat exchanger.
Since pure styrene could polymerize spontaneously in presence of high temperature and high pressure (typically over 6 bar and 125 °C), it is necessary to carry out the distillation process at the lowest possible pressure. This should also be done to avoid the polymerization of the vinyl aromatic chemicals created during the reaction step, considering that the Benzene-Toluene Column operates at a pressure of 1.5 bars while the secondary distillation operation accomplished at the Styrene Column is carried out at 1.0 bar (atmospheric distillation).
Process Mass Balance
Assessment of the mass flows in the referenced document provides the following mass balance details:
- 0.9272 mass units of styrene (purity 99.6%) per mass unit of ethylbenzene (dist. column 2 bottom)
- 2.5 mass units of water per mass unit of ethylbenzene
- 0.9971 mass unit of waste water per mass unit of water consumption (LLV separator bottom)
- 0.0386 mass units of aromatics (benzene / toluene in approx. ratio 1:1) per mass unit of ethylbenzene (dist. column 2 top)
- 0.0270 mass units of fuel gas (hydrogen + methane + ethylene) per mass unit of ethylbenzene (LLV separator top)
- 0.0131 mass units of liquid waste (water + benzene + toluene) per mass unit of ethylbenzene (LLV separator top)
Source: Amaury Pérez-Sánchez et al., 28th Apr 2017, Simulation of the styrene production process via catalytic dehydrogenation of ethylbenzene using CHEMCAD® process simulator, Tecnura, vol. 21, no. 53, pp. 15-31, 2017.