Historical Context
The UOP hydrogen fluoride (HF) alkylation process has a rich development history spanning over eight decades. The foundational HF alkylation technology was developed in UOP laboratories during the late 1930s and early 1940s, initially for producing high-octane aviation fuels from butylenes and isobutane. By the mid-1950s, the increasing demand for sophisticated high-performance automotive engines drove refiners to expand gasoline production while improving motor fuel quality.
The HF alkylation process gained significant momentum by the early 1960s, virtually displacing motor fuel polymerization units for new installations. The technology's importance further increased in the 2000s due to the scheduled phase-out of MTBE and increased emphasis on low-sulfur gasoline production. UOP's reactor and distillation systems evolved through decades of pilot-plant evaluation, engineering development, and commercial operation, resulting in the present concepts in alkylation technology.
Technology Summary and Chemistry
The AlkyPlus process catalytically combines C3-C5 olefins with isobutane to produce isomeric pararaffins in the presence of modified HF catalyst system with ReVAP™ technology, providing enhanced safety characteristics compared to conventional HF systems. The modified catalyst includes proprietary additives that suppress vapor pressure and reduce aerosol formation potential during accidental releases.
Figure 1 - Principal Alkylation Reactions
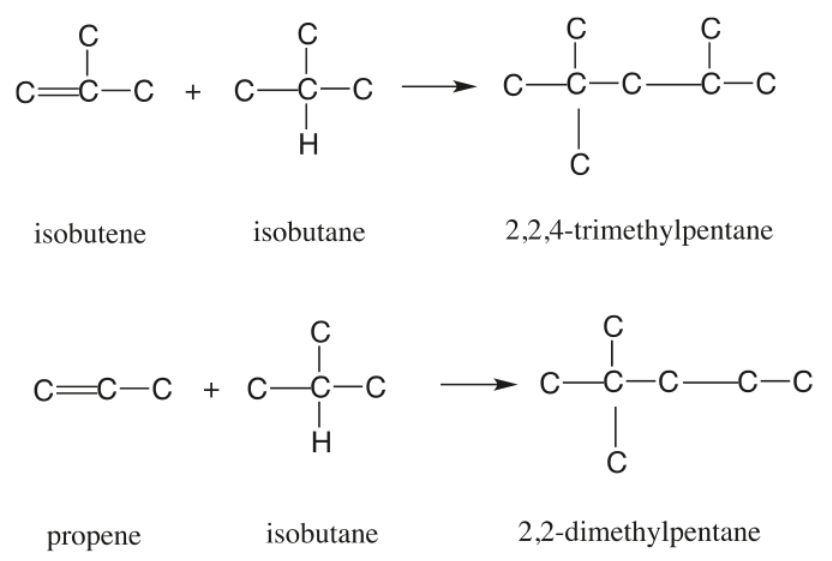
The alkylation reaction mechanism proceeds via the classic carbenium ion pathway, involving initiation, propagation, and isomerization steps. The alkylation reactions follow a carbenium ion mechanism with several key steps:
- Initiation: Generation of tertiary butyl cations that initiate the alkylation reaction chain. HF acid protonates olefins to form carbenium ions.
- Propagation: Tertiary butyl cations react with olefins to form larger carbenium ions, which then abstract hydride from isobutane molecules, generating isoparaffins and regenerating tertiary butyl cations.
- Isomerization: Critical for producing high octane quality, particularly from 1-butene feeds. The isomerization of 1-butene to 2-butene reduces dimethylhexane production (RON 55-76) and increases trimethylpentane (isooctane) formation.
- Hydrogen Transfer: Most pronounced with propylene feeds, this reaction produces butylene and propane, with the butylene subsequently alkylating to form trimethylpentane.
Secondary reactions such as hydrogen transfer, polymerization, isomerization, and destructive alkylation also occur, resulting in formation of secondary products both lighter and heavier than primary products. Not all secondary reactions are undesirable; for example, they make possible the formation of isooctane from propylene or amylenes.
Detailed Process Description with Processing Steps
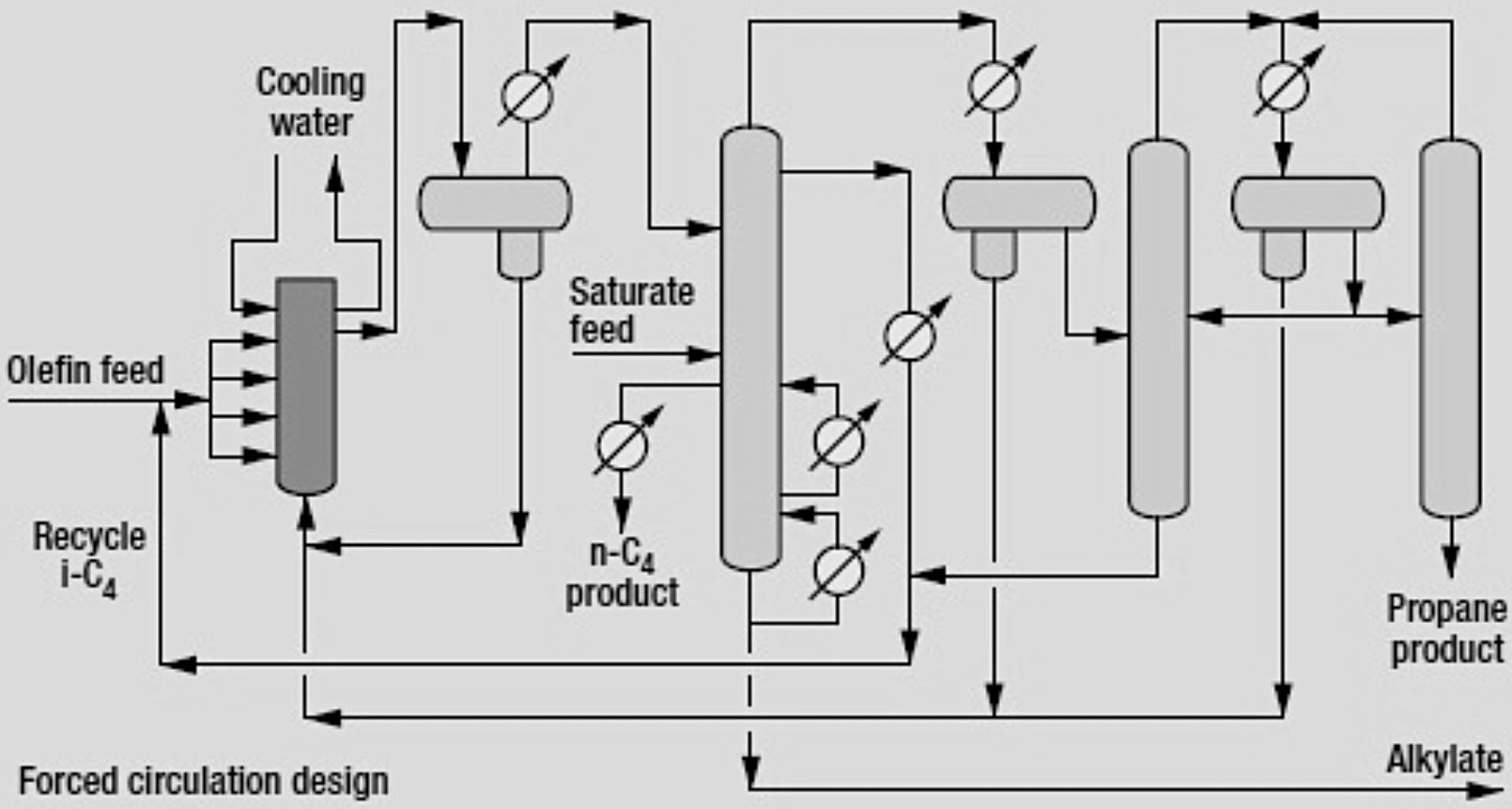
The AlkyPlus™ process follows the proven UOP isothermal reactor design with several enhancements. The process consists of five main sections:
- Feed Treating Section:
Feed treatment includes the following components:
- Alkylation feedstock (C3, C4, C5 olefins or mixtures) undergo treating to remove sulfur and water
- Feed drying systems remove water to prevent catalyst degradation
- Optional oxygenate removal unit (ORU) for processing MTBE/TAME raffinates
- Selective hydrogenation option for butylene feedstock to reduce catalyst regeneration requirements, catalyst (acid) consumption, and increase alkylate octane by isomerizing 1-butene to 2-butene
- Enhanced Isothermal Reactor System:
In an ideally designed and operated system, primary reactions should predominate, but not to the complete exclusion of secondary ones. The optimum combinations of plant economy, product yield, and quality are achieved with the reaction system operating at cooling-water temperature, an excess of isoparaffin, contaminant-free feedstocks, and vigorous, intimate acid-hydrocarbon contact:
- Reactor Design: Enhanced isothermal reactor designed to minimize catalyst inventory while ensuring efficient hydrocarbon-acid contacting. UOP offers two reactor configurations, both ensuring efficient contacting and mixing of hydrocarbon feed with the acid catalyst:
- Gravity flow design: Provides good mixing without the need for a catalyst circulation pump
- Forced circulation design: Reduces acid inventory and reaction vessel elevations
- Temperature Control: Continuous heat removal in the reaction zone reduces peak temperatures and enhances product selectivity
- Mixing System: Efficient dispersion of hydrocarbon feed with acid catalyst through specially designed feed distributors
- Heat Exchange: Effective heat transfer utilizing available cooling water supply; undesirable reactions are minimized by the continuous removal of heat of reaction in the reaction zone itself.
- Operating Conditions:
- Temperature: Cooling water temperature (typically 35-45°C)
- Pressure: Moderate pressure operation
- Isobutane to olefin ratio: Optimized for maximum alkylate quality
- Acid inventory: Minimized through high heat-transfer rates and efficient circulation
- Settling Section, Product Separation and Treatment:
Settling, separation and product treatment section include the following components:
- Acid-hydrocarbon separation in settling vessels
- Gravity separation with settled acid returned to reactor through catalyst cooler
- Hydrocarbon phase containing propane, recycled isobutane, normal butane, and alkylate sent to fractionation
- Main Fractionator: Separates hydrocarbon products into distinct streams
- Isostripper:
- The modern isostripper recovers relatively high-purity, virtually acid-free isobutane as a sidecut that is recycled to the reactor
- A small rectification section on top of the modern isostripper provides for more efficient propane rejection
- HF Stripper: Removes HF from product streams
- Propane Recovery: High-purity propane through HF stripper, defluorinator, and potassium hydroxyde (KOH) treatment
- Product Treatment: Defluorination and KOH treatment for all products
- Acid Regeneration:
The on-site acid catalyst regeneration removes heavy oils (tars) from catalyst and the additive recovery system handles ReVAP™ components. This technique virtually eliminates the need for an acid regenerator and, as a result, acid consumption has been greatly reduced. It also eliminates polymer disposal. The acid regenerator has been retained in the UOP design only for start-ups or during periods when the feed has abnormally high levels of contaminants, such as sulfur and water.
- When the acid regenerator is in service, a drag stream off the acid circulation line at the settler is charged to the acid regenerator, which is refluxed on the top tray with isobutane.
- Heat Source Configuration:
- C3-C4 Alkylation units: Superheated isobutane from the depropanizer sidecut vapors provides heat to the bottom of the regenerator
- C4 Alkylation units: Sidecut vapors from the HF stripper bottoms serve as the stripping medium to the acid regenerator
- Regenerated HF acid is combined with the overhead vapor from the isostripper and sent to the cooler.
- Neutralization Section:
UOP has designed the neutralization section to minimize the amount of additional effluents such as offensive materials and undesirable by-products. Releasing acid-containing vapors to the regular relief-gas system is impractical because of corrosion and odor problems as well as other environmental and safety concerns.
- System components: KOH mixing tank, circulating pumps, scrubber, KOH regeneration tank.
- All acid vents and relief valves are piped to this relief section.
- Relief-gas scrubber: Gases pass up through the scrubber and are contacted by a circulating KOH solution to neutralize the HF acid
- Flaring: After the neutralization of the acid, the gases can be safely released into the refinery flare system
- KOH Regeneration: The KOH is regenerated on a periodic basis in the KOH regeneration tank by using lime to form calcium fluoride (CaF₂) and KOH
- The CaF₂ settles to the bottom of the tank and is directed to the neutralizing basin, where acidic water from acid sewers and small amounts of acid from the process drains are treated.
- Lime is used to convert any fluorides into calcium fluoride before any waste effluent is released into the refinery sewer system.
Safety and Mitigation Features
The AlkyPlus™ process incorporates multiple safety enhancements:
Active Mitigation:
- Improved volatility suppression systems
- Enhanced detection and monitoring systems
- Rapid response mitigation equipment
Passive Mitigation:
- Pre-blended catalyst reducing transportation risks
- Reduced aerosol formation characteristics
- Lower catalyst inventory requirements
Technology Performance
Yields and Product Properties
The AlkyPlus™ process delivers competitive yields and high-quality alkylate products:
| Olefin Feed |
Isobutane Consumed (vol/vol) |
C5+ Alkylate Produced (vol/vol) |
API Gravity |
RON Clear |
MON Clear |
RVP (psia) |
| C3= |
1.4 |
1.8 |
70.7 |
91.0 |
89.5 |
3.0 |
| C4= |
1.2 |
1.8 |
69.9 |
96.0 |
94.0 |
2.7 |
| C5= |
1.3 |
2.0 |
69.9 |
90.0 |
89.0 |
0.45 |
Alkylate Quality Characteristics
The AlkyPlus™ process produces alkylate with superior environmental and performance characteristics:
- Ultra Low Sulfur: Essentially sulfur-free product
- No Aromatics: Clean-burning characteristics
- High Octane: Research octane numbers 90-96 clear
- No Olefins: Stable blending component
- Low Vapor Pressure: Assists in gasoline pool RVP control
Catalyst Efficiency:
- HF acid consumption rate is less than 1/100th the rate for sulfuric alkylation units (some sources cite 1/200th)
- No expensive solid catalysts required
- Simple on-site catalyst regeneration
Operational Efficiency:
- No mechanical agitation required
- No refrigeration equipment needed ((unlike sulfuric alkylation units which require refrigeration to maintain low reactor temperature)
- No compression requirements
- High on-stream availability through elimination of compressors and rotating equipment in reactor
Energy Integration:
- Efficient heat transfer with cooling water utilization
- Heat integration opportunities in fractionation section
- Steam generation potential from reaction heat
Process Flexibility
The AlkyPlus™ technology provides enhanced operational flexibility:
- Wide range of olefin feedstocks (C3-C5)
- Ability to process MTBE/TAME raffinates with appropriate pretreatment
- Revamp capabilities for existing HF alkylation units
- Scalable design for various capacity requirements
Commercial Experience and Process Scale
Global Market Position
UOP maintains its position as the leading licensor of alkylation technology with more than 210 licensed units worldwide as of 2016. The company's alkylation portfolio has evolved to include several technology variants addressing different market needs and safety requirements.
Technology Evolution and Deployment
The AlkyPlus™ process represents the latest evolution in UOP's alkylation technology development, building upon decades of commercial experience. The technology incorporates lessons learned from extensive commercial operations and addresses current industry challenges related to safety, environmental compliance, and economic optimization.
Commercial Scale Applications:
- New grassroots installations
- Revamp projects for existing HF alkylation units requiring major modifications
- Capacity expansion projects where full reactor/settler replacement is required
- Units requiring enhanced safety profile competitive with alternative technologies
Implementation Considerations
Revamp Opportunities:
The AlkyPlus™ process solution is particularly suitable for existing HF alkylation units requiring major modifications to achieve refinery goals. The technology can be implemented when full reactor/settler replacement is required for capacity expansion, providing necessary performance upgrades coupled with reduced-risk profile.
Project Economics:
The technology licensor claims that the AlkyPlus™ solution remains economically attractive for alkylate production, offering competitive capital and operating costs while providing enhanced safety characteristics. The technology's ability to process various feedstocks and integrate with existing infrastructure provides additional economic benefits for refiners.
References
- PennState College of Earth and Mineral Sciences > FSC 432: Petroleum Refining > Lesson 8: Catalytic Conversion Processes Part 2 > Alkylation > UOP HF Alkylation Process.
- Honeywell UOP. 2016 UOP LLC Information Bulletin: UOP AlkyPlus™ Alkylation Process Solution.
- Hassan ElBanhawi website. Alkylation Process: UOP Alkyplus Process.
- Jack. Jan 24, 2019. HF Alkylation Process by UOP. Oil & Gas Process Engineering.
- Dr. Semih Eser © Penn State, FSC 432 PETROLEUM PROCESSING, Alkylation, image licensed under CC BY-NC-SA 4.0.