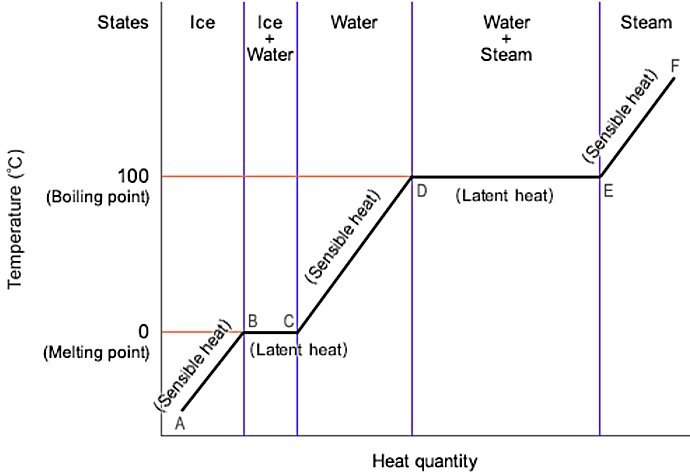
Heat Content
Heat content refers to the instantaneous internal energy of a system associated with:
- sensible heat: the microscopic motions (kinetic energy) of the particles in a material, which increases with the temperature.
- latent heat: the interactions (potential energy) of the particles in a material, changing with phase change such as melting (fusion), freezing, vaporization (boiling), condensation, sublimation, deposition).
Heat content is particularly relevant for the efficiency of heat transfer materials in heating and cooling applications and is quantified by parameters like specific heat capacity, thermal conductivity, and latent heat of fusion/vaporization.
Common Heat Transfer Materials in Heating and Cooling Processes
| Fluid Type |
Examples |
Key Properties |
Applications |
| Water |
Pure, deionized |
High Cp (4.18 kJ/kg°C) |
HVAC, electronics cooling |
| Dielectric Fluids |
Fluorinert™, PAO oils |
Non-conductive, low Cp |
Power electronics, aerospace |
| Molten Salts |
Nitrate/nitrite blends |
High thermal stability (>400°C) |
Concentrated solar power (CSP) |
| Synthetic Oils |
Silicone, aromatic hydrocarbons |
Low vapor pressure, high-temperature use |
Industrial reactors, molding |
| Steam |
Saturated/superheated |
High latent heat (2,260 kJ/kg at 100°C) |
Power generation, process heating |
| Refrigerants |
Ammonia (R717), CO2 (R744), HFCs |
Low boiling points, high latent heat |
Industrial refrigeration, heat pumps |
| Brine Solutions |
Glycol-water, calcium chloride |
Freeze protection (-40°C), moderate Cp |
Food processing, ice rinks |
| Cryogens |
Liquid nitrogen (-196°C), LNG |
Ultra-low temps, high latent heat |
Medical cryopreservation, LNG cold recovery |
| PCMs (Cold) |
Paraffin, salt hydrates |
Phase change at low temps (e.g., -20°C) |
Cold storage, portable coolers |
Key Parameters
- Specific Heat Capacity (Cp):
Energy required to raise 1 kg of fluid by 1°C (e.g., water: 4.18 kJ/kg°C, PAO oil: 2.1 kJ/kg°C).
- Latent Heat (L):
Energy absorbed/released during phase change (e.g., steam: 2,260 kJ/kg at 100°C, ammonia: 1371.2 kJ/kg at -33.5°C and Standard pressure (1.013 bar)).
- Thermal Conductivity (k):
Ability to conduct heat (e.g., water: 0.6 W/m·K, molten salts: 0.5 W/m·K).
- Viscosity & Freezing Point:
Critical for cold fluids to avoid solidification (e.g., glycol-water: -35°C).
Heat/Cold Energy Content Calculation
Energy content depends on whether heat transfer involves sensible or latent mechanisms:
- Sensible Heat/Cold (No Phase Change):
Q = m⋅Cp⋅ΔT
where m is Mass (kg), Cp is specific heat capacity (kJ/kg°C), and ΔT is Temperature change (°C)
Example: Cooling 10 kg of water from 80°C to 20°C:
Q = 10 kg⋅4.18 kJ/kg°C⋅(80−20) = 2,508 kJ
- Latent Heat/Cold (Phase Change)
Q = m⋅L
where m is mass and L is Latent heat of vaporization (kJ/kg)
Example: Vaporizing 2 kg ammonia
Q = 2⋅1,371 = 2,742 kJ
- Steam (Combined Sensible + Latent):
-
Sensible Heat (Liquid to Boiling Point):
Qsensible = m⋅Cp⋅ΔT
Example: Heating 1 kg water from 25°C to 100°C:
1 kg⋅4.18 kJ/kg°C⋅75 = 313.5 kJ
-
Latent Heat (Vaporization):
Qlatent = m⋅L
Example: Vaporizing 1 kg water at 100°C:
1 kg⋅2,260 kJ/kg = 2,260 kJ
-
Total Heat Content (Saturated Steam):
Qtotal = 313.5 + 2,260 = 2,573.5 kJ/kg
Usage Context
| Fluid |
Typical Applications |
| Steam |
Power plants (turbines), district heating, sterilization, food processing |
| Molten Salts |
CSP plants (400°C storage), industrial high-temp processes |
| Glycol-Water |
Solar thermal systems, engine cooling (freeze protection) |
| Dielectric Fluids |
Immersion cooling for EV batteries, data centers |
| Ammonia (R717) |
Large-scale refrigeration (food processing, warehouses) |
| CO2 (R744) |
Supermarket freezers, heat pumps (low global warming potential) |
| Glycol-Water |
Ice rinks, brewery cooling, HVAC chillers |
| Liquid Nitrogen |
Cryogenic freezing, medical sample storage, superconductors |
| LNG Cold Energy |
Waste cold recovery for power generation, air separation plants |
| PCMs |
Passive heating and cooling systems in buildings |
Performance Comparison
Property
Product |
Max Temp |
Energy Density |
Phase Change |
Infrastructure |
Typical COP* |
| Steam |
100–600°C (pressurized) |
Very High (latent + sensible) |
Yes |
High-pressure piping |
N/A |
| Water |
20–200°C |
Moderate |
No |
Low-pressure pipes |
N/A |
| Thermal Oil |
300–400°C |
Low-Moderate |
No |
Pumps/heat exchangers |
N/A |
| Molten Salts |
250–600°C |
High (sensible) |
No |
High-temp vessels |
N/A |
| Ammonia (R717) |
-33°C (evap.) |
High (latent) |
Yes |
Cryogenic storage |
3–5 |
| Liquid Nitrogen |
-196°C |
Extreme (latent) |
Yes |
Cryogenic storage |
0.2–0.3 (cryogenic) |
*Coefficient of Performance (COP) for refrigeration cycles.
Advantages and Challenges
Steam
- Pros:
- Highest energy density via latent heat (5–10x more efficient than water).
- Precise temperature control via pressure adjustment.
- Cons:
- Corrosion/scale risks without water treatment.
- High-pressure systems require rigorous safety measures.
Other Heat Transfer Fluids
- Water/Glycol: Low cost but limited to moderate temperatures.
- Molten Salts: Ideal for high-temp storage but suffer from solidification risks.
- Dielectric Fluids: Safe for electronics but have low thermal conductivity.
Cold Transfer Fluids
- Pros:
- Enable sub-zero cooling for critical applications (e.g., vaccines, superconductors).
- Waste cold recovery improves energy efficiency (e.g., LNG terminals reduce energy use by 20–30%).
- Cons:
- Cryogens require specialized insulation and handling.
- Refrigerants like HFCs contribute to global warming if leaked.
Example Systems
- Industrial Steam Boiler:
- Converts 1 kg water to 10 bar steam (180°C), releasing 2573.5 kJ/kg.
- Condenses in heat exchangers for process heating.
- LNG Regasification:
- LNG is vaporized to natural gas at import terminals, releasing ~830 kJ/kg of cold energy.
- This energy is reused for air separation, cold storage, or electricity generation:
1 tonne LNG regasification releases 830 MJ cold energy, used to chill 5,000 kg water by 40°C.
- Industrial Gas Production:
- Cold from liquid nitrogen production is recycled to chill water or pre-cool gases.
- Ammonia evaporates at -33°C, absorbing 1370 kJ/kg.
- Solar Power Plant:
- Molten salts (60% NaNO3/40% KNO3) store 1.5 kJ/kg°C sensible heat at 400°C.
- Delivers energy via steam turbines after heat exchange.
- EV Battery Cooling:
- PAO oil (Cp = 2.1 kJ/kg°C) absorbs heat from battery packs via forced convection.
By leveraging these properties, engineers select heat transfer materials to optimize thermal management across industries, balancing energy density, temperature range, and system complexity. Steam remains unparalleled for high-energy-density applications, while newer fluids address niche demands like electronics cooling or renewable energy storage.
References
Perplexity A.I. Deepsearch assisted description, 31st Mar 2025.