Product
- Main Product
- Sodium Hypochlorite
- Segment
- Chemicals
- Main-Family
- Inorganics
- Sub-Family
- Inorganic Chlorine Compounds
- Physical State
-
Solid
- Product
- Chlorine Bleach
- Production
- Global Production Production Map
-
Sodium hypochlorite is most often encountered as a pale greenish-yellow dilute solution referred to as chlorine bleach, which is a household chemical widely used (since the 18th century) as a disinfectant and bleaching agent. In solution, the compound is unstable and easily decomposes, liberating chlorine, which is the active principle of such products. Sodium hypochlorite is still the most important chlorine-based bleach.
Household bleach is, in general, a solution containing 3–8% sodium hypochlorite, by weight, and 0.01–0.05% sodium hydroxide; the sodium hydroxide is used to slow the decomposition of sodium hypochlorite into sodium chloride and sodium chlorate.
Source: Wikiedia, Sodium hypochlorite.
Identifiers
-
 CAS Number
CAS Number
- 7681-52-9
-
 EC Number
EC Number
- 231-668-3
-
 ECHA InfoCard
ECHA InfoCard
- 100.028.790
-
 IUPAC Name
IUPAC Name
- Sodium Hypochlorite
-
 PubChem ID
PubChem ID
- 23665760
Chemical Data
- Chemical Formula
-
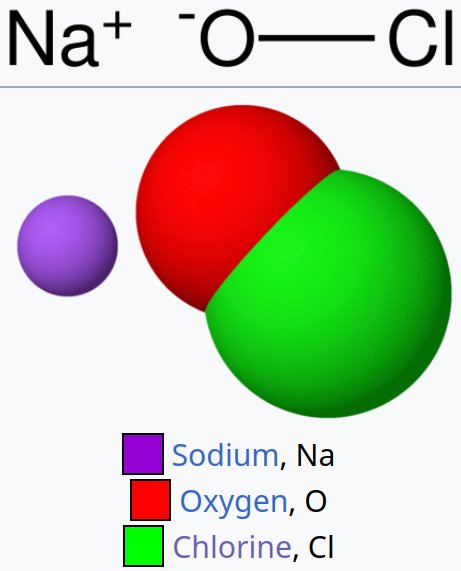
NaOCl
- Molecular Weight (g/mol)
- 74.442
- Sulfur Content (wt%)
- 0
- Specific Gravity
- 1.11
Properties
- Default
- Status
- A
System Info
- Update by
-
 Kokel, Nicolas
Kokel, Nicolas - Updated
- 12/31/2024 5:39 PM
- Added by
-
 Kokel, Nicolas
Kokel, Nicolas - Added
- 12/31/2024 5:08 PM

No Services yet available.
Enquire in Solutions how we can help you.
Product Communicator
| Title | Date | |
|---|---|---|

|
12/31/2024 |