Since crude oil generally contains only 10-40% of hydrocarbon constituents in the gasoline range, refineres typically use an FCCU to convert high molecular weight hydrocarbons into smaller and more volatile compounds, which are then converted into liquid gasoline-size hydrocarbons. Byproducts of the FCC process also creates other low molecular-weight alkenes and iso-paraffin molecules which are not desirable.[1]
Alkylation transforms these byproducts into larger iso-paraffin molecules or alkylate, a high-octane gasoline blending component. Alkylate product is a mixture of branched paraffinic hydrocarbons of gasoline boiling range (mostly isoheptane and isooctane). Alkylate has a Motor Octane (MON) of 90-95 and a Research Octane (RON) of 93-98. Because of its high Octane and low vapor pressure, alkylate is considered an excellent blending component for gasoline.[2],[3]
In the refinery, isobutane is alkylated with isobutene in the presence of a strong acid catalyst, either sulfuric acid or hydrofluoric acid. Depending on the acid used, the process is referred to as a SAAU or a HFAU. However, oil refinery employees may simply refer to the unit as the Alkyl or Alky Unit. Propylene present in the feed in small concentration also reacts with isobutane to form isoheptane.[2],[3]
Figure 1 - Principal Alkylation Reactions[4]
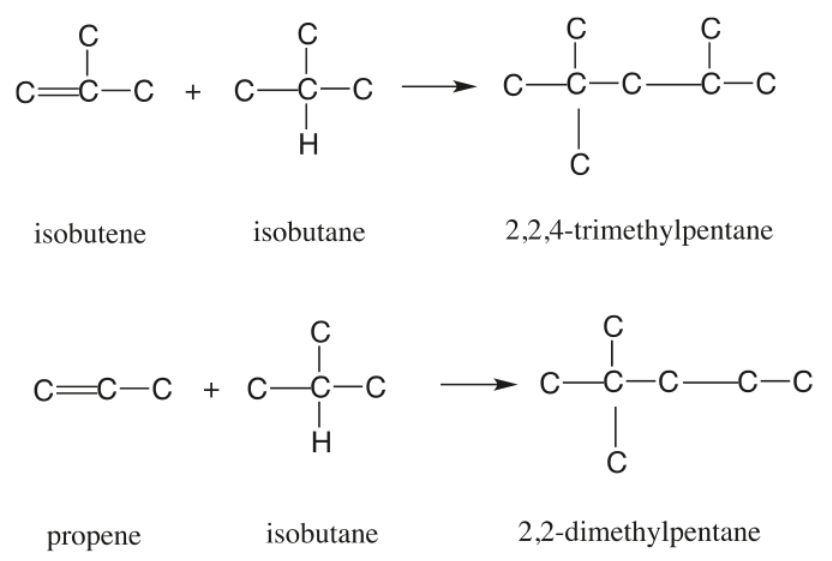
Under reaction conditions unfavorable to alkylate formation, propylene may also polymerize to form the undesirable product polypropylene. Amylenes can undergo a similar reaction to form alkylate, but since amylenes have a high octane number to start with, their conversion to alkylate is not as advantageous, as in the case of butylenes. Another side Reaction that can negatively affect the formation of alkylate is ester formation by the reaction of olefins with sulfuric acid.[2]
Alkylate is a component of choice in gasoline, because it is free of aromatics and olefins. About 11% of the gasoline winter pool in the U.S. is made up of alkylate. In the gasoline summer pool, the content of alkylate can be as high as 15% because lower Reid vapor pressure (RVP) reduces the possibility to blend butane.[1]
References
- Alkylation Unit, Wikipeda
- Alkylation, Brewiki
- REFINING - ALKYLATION, techSTAR
- Dr. Semih Eser © Penn State, FSC 432 PETROLEUM PROCESSING, Alkylation, image licensed under CC BY-NC-SA 4.0










