Polystyrene (PS) is a synthetic aromatic Hydrocarbon Polymer made from of the aromatic Hydrocarbon Styrene.
In chemical terms, Polystyrene is a long chain Hydrocarbon wherein alternating Carbon centers are attached to Phenyl Groups (a derivative of Benzene). Polystyrene's chemical formula is (C8H8)n; it contains the chemical elements Carbon and Hydrogen.
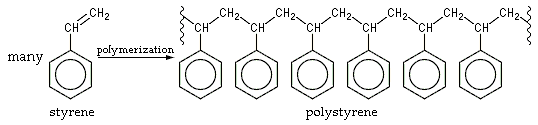
Polystyrene is an Addition Polymer that results when Styrene Monomers polymerize (interconnect). In the Polymerization, the Carbon-Carbon π Bond of the Vinyl Group is broken and a new Carbon-Carbon σ bond is formed, attaching to the Carbon of another Styrene Monomer to the Chain. Since only one kind of Monomer is used in its preparation, it is a Homopolymer (Fig. 1). The newly formed σ bond is stronger than the π bond that was broken, thus it is difficult to Depolymerize Polystyrene. About a few thousand Monomers typically comprise a Chain of Polystyrene, giving a molar mass of 100,000–400,000 g/mol.
Figure 1 - Polystyrene Formation
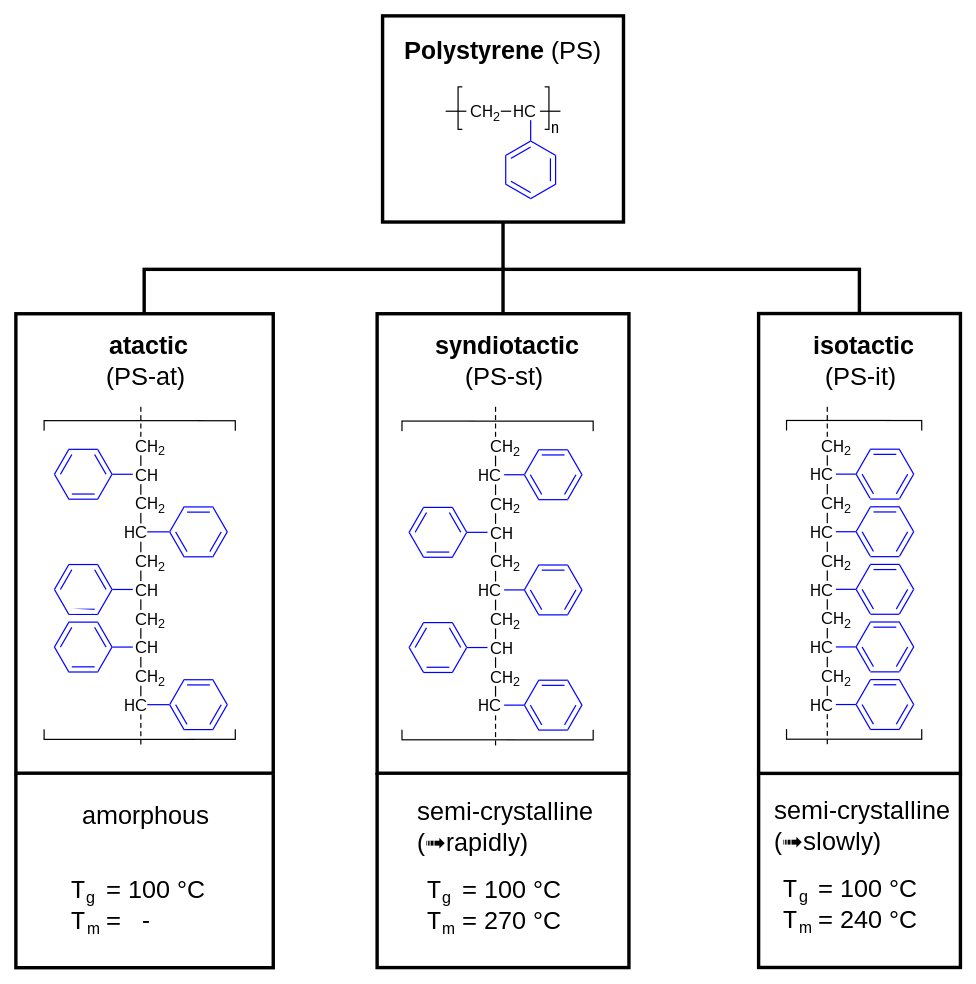
The relative stereochemical relationship of consecutive Phenyl Groups determines the Tacticity, which affects various physical properties of the Material. In Polystyrene, tacticity describes the extent to which the Phenyl Group is uniformly aligned (arranged at one side) in the Polymer Chain. Tacticity has a strong effect on the properties of the Plastic:
- Standard Polystyrene is atactic, which Polymerization is initiated with Free Radicals.
- The Diastereomer where all of the phenyl groups are on the same side is called isotactic Polystyrene, which is not produced commercially.
- Ziegler–Natta Polymerization can produce an ordered syndiotactic Polystyrene with the Phenyl Groups positioned on alternating sides of the Hydrocarbon Backbone.
Figure 2 - Polystyrene Tacticity
Source: Wikipedia, Polystyrene

















