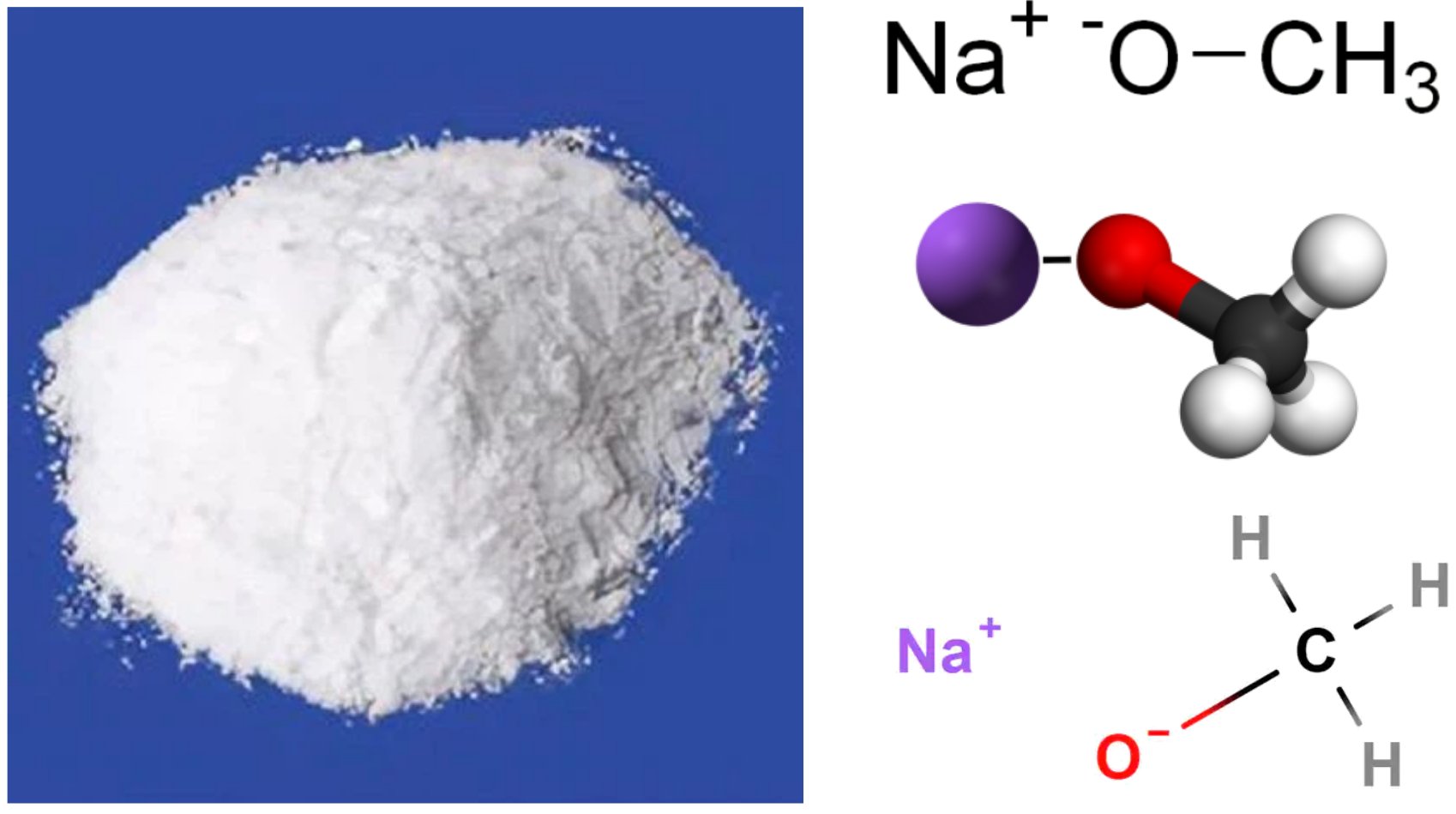
Sodium methoxide (also known as sodium methylate) is an organometallic compound with the molecular formula CH₃ONa or NaOCH₃. This compound represents the sodium salt of methanol, formed when methanol undergoes deprotonation.
Molecular Structure
Sodium methoxide consists of a sodium cation (Na⁺) and a methoxide anion (CH₃O⁻). The compound exhibits a hexagonal crystal structure and is classified as a strong base with a pKa of approximately 15.17.
Chemical Reactivity
The compound demonstrates several critical reactivity patterns:
- Hygroscopic Nature: Rapidly absorbs moisture from air, leading to decomposition
- Water Sensitivity: Reacts violently with water according to: NaOCH₃ + H₂O → NaOH + CH₃OH
- Air Sensitivity: Decomposes in air above 126.6°C, forming sodium hydroxide and methanol
- Auto-ignition Temperature: >50°C, making it a fire hazard
Solubility Characteristics
- Soluble in: Methanol, ethanol, and other polar alcohols
- Insoluble in: Water (reacts violently), benzene, toluene, and hydrocarbons
- Miscible with: Fats and esters due to its amphiphilic nature
Manufacturing Process
The manufacturing of sodium methoxide in industrial settings employs multiple production routes
1. Sodium Hydroxide-Methanol Method (primary method - 85%+ of global production)
NaOH + CH₃OH ⇌ CH₃ONa + H₂O
- Key challenge: Unfavorable equilibrium constant requiring continuous water removal
- Industrial solution: Reactive distillation with azeotropic water separation
- Typical conditions: 120-175°C, 0.3-2.5 MPa pressure
- Yield: 98-99.5% with modern high-pressure processes
2. Metallic Sodium-Methanol Method (Secondary method - 10-15% of production, being phased out)
2 Na + 2 CH₃OH → 2 NaOCH₃ + H₂↑
- Advantages: High purity product, simple process, complete conversion
- High costs: Metallic sodium raw material costs 8-12 times more than sodium hydroxide
- Handling complexity: Metallic sodium requires specialized storage and handling
3. Sodium Amalgam Process (Minor method - <5% combined)
Na(Hg) + CH₃OH → CH₃ONa + Hg + ½H₂↑
- Applications: Limited to facilities with mercury cell technology
- Environmental concerns: Mercury contamination issues
Commercial Product Forms
The compound is available in multiple commercial forms:
- Solid Powder: 98-99% purity, typically packaged under nitrogen atmosphere
- Solution in Methanol:
- 25% concentration (5.4M)
- 28-30% concentration (standard industrial grade)
- Color: Pure solid is white; solutions may appear colorless to slightly yellow
Major Applications
Biodiesel Production
The primary industrial application of sodium methoxide is as a catalyst in biodiesel production through transesterification. In this process:
- Triglycerides (vegetable oils or animal fats) react with methanol
- Sodium methoxide catalyzes the transesterification to fatty acid methyl esters (FAME)
- Typical catalyst concentration: 0.5-1.0% by weight of oil
- Reaction conditions: 60-65°C, atmospheric pressure
Pharmaceutical Industry
Sodium methoxide serves multiple roles in pharmaceutical synthesis:
- API Synthesis: Critical reagent for active pharmaceutical ingredients
- Methylation Agent: Introduces methyl groups into organic molecules
- Base Catalyst: Facilitates condensation and deprotonation reactions
- Specific Applications: Production of vitamin A, vitamin B₁, sulfadiazine, and other medications
Chemical Synthesis
In organic chemistry laboratories and industrial processes:
- Strong Base: Non-aqueous basic conditions for sensitive reactions
- Nucleophile: Attacks electrophilic centers in substitution reactions
- Polymerization Initiator: Initiates anionic addition polymerization with ethylene oxide
- Ester and Ether Synthesis: Key reagent in alkylation and condensation reactions