Main-Product
- Product
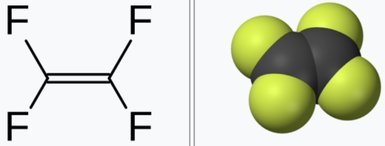
- Tetrafluoroethylene
- Segment
- Chemicals
- Main-Family
- Unsaturated Monomers
- Sub-Family
- Functional monomers
- Link
-

- #PS601
Description
Your insights will be shown here
Product Communicator
(*=Default)| Product | Title | Date | |
|---|---|---|---|

|
8/3/2024 |
| Products (Quick Access) | Abbr. | Default |
|---|---|---|
| Tetrafluoroethylene | TFE |
Settings
- Status
- A
- Unit of Measure
- Metric Ton
- Physical State
-
Gas
Building Block / Value Chain Info
- Value Chain-I
- Carbon Monoxide
Content provided by
| Transaction | Name | Date |
|---|---|---|
| Modified by |
|
8/4/2024 2:52 PM |
| Added | 8/3/2024 9:12 AM |