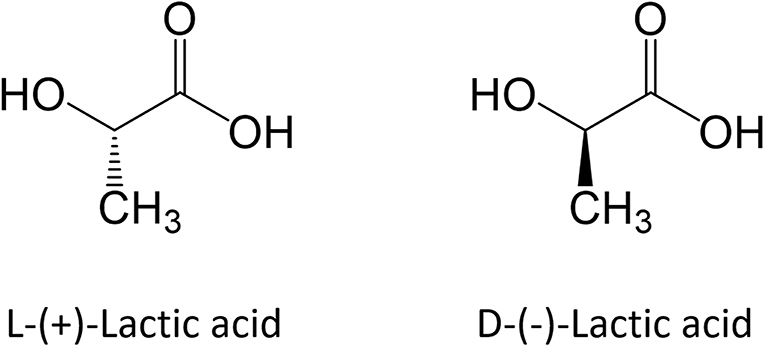
Description
Lactic Acid, also known as 2-Hydroxypropanoic Acid, is an Organic Acid. It has a molecular formula CH3CH(OH)COOH. It is white in the solid state and it is miscible with Water. When in the dissolved state, it forms a colorless solution. Lactic acid is an Alpha-Hydroxy Acid (AHA) due to the presence of a Hydroxyl Group adjacent to the Carboxyl Group. It is used as a Synthetic Intermediate in many organic synthesis industries and in various biochemical industries.[1],[2]
Chemical Synthesis
Racemic Lactic Acid is synthesized industrially by reacting Acetaldehyde with Hydrogen Cyanide (1) and hydrolysing the resultant Lactonitrile at low pH in order to yield Lactic Acid (2). When Hydrolysis is performed by Hydrochloric Acid, Ammonium Chloride forms as a By-product (2). Lactic Acid is subsequently converted to Methyl Lactate (3) through Esterification and eventually recovered and purified by Distillation. Lactic Acid and Methanol are obtained through Hydrolysis from Lactate; Methanol is recycled in step (3).[1],[2] This kinetic pathway leads to a Racemic Mixture of L-(+)-Lactic Acid and D-(-)-Lactic Acid.[2] Tthe Japanese company Musashino is one of the last big manufacturers of Lactic Acid by this route.[1]
| |
CH3CHO + HCN → CH3CH(OH)CN |
(1)
|
| |
CH3CH(OH)CN + 2H2O + HCl → CH3CH(OH)COOH + NH4Cl |
(2)
|
| |
CH3CH(OH)COOH + CH3OH ↔CH3CH(OH)COOCH3 + H2O
|
(3) |
Bacterial Fermentation of Sugars
Bacterial Fermentation of Sugar Solutions is currently the most employed Process; this Process leads to high yields and, depending on the chosen type of Bacteria, it allows obtaining one given Stereoisomer or the Racemic Mixture. Lactic Acid Bacteria produce either l(+), d(−), or racemic dl Lactic Acid. Molds produce only l(+) Lactic Acid.[2],[3] It is estimated that about 90% of the total Lactic Acid produced worldwide is currently obtained with this procedure.[2]
As a starting Material for Industrial Fermentative Production of Lactic Acid, almost any Carbohydrate Source containing C5 (Pentose Sugar) and C6 (Hexose Sugar) can be used. Pure Sucrose, Glucose from Starch, Raw Sugar, and Beet Juice are frequently used. Lactic Acid producing Bacteria can be divided in two classes: Homofermentative Bacteria like Lactobacillus casei and Lactococcus lactis, producing two moles of Lactate from one mole of Glucose, and Heterofermentative Species producing one mole of Lactate from one mole of Glucose as well as Carbon Dioxide and Acetic Acid/Ethanol.[1]
References
- Wikipedia, Lactic Acid
- Casalini T, Rossi F, Castrovinci A and Perale G (2019) A Perspective on Polylactic Acid-Based Polymers Use for Nanoparticles Synthesis and Applications. Front. Bioeng. Biotechnol. 7:259. doi: 10.3389/fbioe.2019.00259
- J.H. Litchfield, Encyclopedia of Microbiology (Third Edition), 2009