Main-Product
- Product
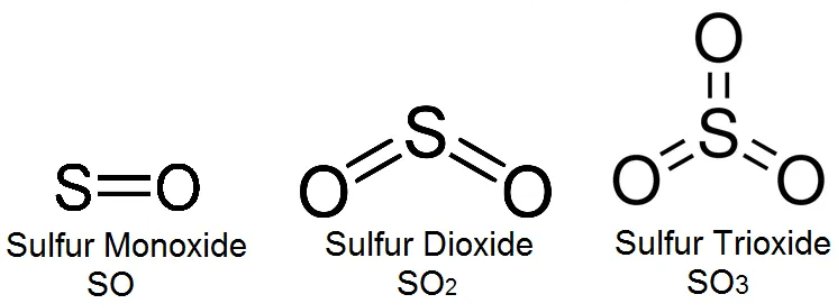
- Sulfur Oxides
- Segment
- Refined Products
- Main-Family
- Refinery Gases
- Sub-Family
- Gaseous Sulfur Compounds
- Link
-

- #PS399
Description
Your insights will be shown here
Product Communicator
(*=Default)| Product | Title | Date | |
|---|---|---|---|

|
12/2/2025 | ||

|
6/17/2025 | ||

|
1/1/2025 |
| Products (Quick Access) | Abbr. | Default |
|---|---|---|
| Sulfur Oxides | SOx | |
| Sulfur Dioxide | SO₂ | |
| Sulfur Trioxide | SO3 |
Settings
- Status
- A
- Unit of Measure
- Metric Ton
- Physical State
-
Gas
Content provided by
| Transaction | Name | Date |
|---|---|---|
| Modified by |
|
1/1/2025 9:18 PM |
| Added | 6/10/2022 6:57 PM |