🇫🇷 France, the future El Dorado of hydrogen?
- Product
- White Hydrogen
- Message Category
-
Translation of an article published in French language on 'La Sélection du Jour' by Janus Maat on 12th March 2024.
The Largest Global Deposit of Hydrogen Located Underground in France! The largest global deposit of hydrogen is located underground in France! Last year, in 2023, French geologists drilled the Lorraine soil in search of methane, a gas widely used in industry. According to their analyses, they thought they would find 370 billion cubic meters there, which represents 8 years of global gas consumption. A probe specially designed for this type of drilling, patented in April 2023, then began to dig at a depth of more than 1,000 meters in a well on the commune of Folschviller, 50 kilometers from Metz. Their calculations seem good. They find methane, and pure methane at that! Bingo. But how surprised the French geologists were when they realized that the more they dug, the more the presence of an even more precious gas became significant: hydrogen, or dihydrogen to be more precise, noted H2.
At 800 meters, the quantity of H2 went from 1% to 6%, then to 15% at 1,100 meters... According to their estimates, at 3,000 meters deep, the hydrogen content would even be 90%, which would make the Lorraine deposit a true reservoir of more than 50 million tonnes of natural hydrogen, representing the greatest global concentration of this so-called "white" hydrogen, corresponding to half of global production of "gray" hydrogen.
White? Gray? What do these color codes mean? To better understand them, we need to revisit the production method of hydrogen. The latter is a flammable gas, containing an enormous quantity of energy when placed in contact with oxygen. But it is also very volatile, and any natural source evaporates instantly. It must therefore be synthesized either from a carbon source (black hydrogen) or from methane (gray hydrogen). How to do it?
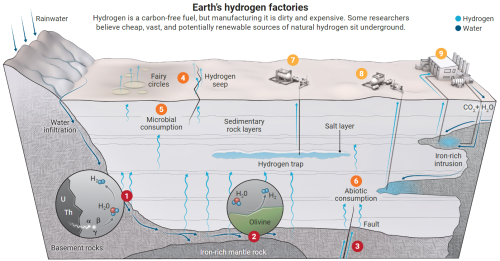
The Earth and its hydrogen productions (source: C. BICKEL/SCIENCE (DATA) GEOFFREY ELLIS/USGS)
The chemical composition of methane is written CH4: meaning one carbon atom C well surrounded by four hydrogen atoms (H4). It is therefore necessary to break this group of five by force to recover dihydrogen (H2). But this forceful action doesn't happen without a hitch. Dihydrogen (H2) combines by burning with oxygen to form water (H2O), a perfectly clean process since only water is produced following the combustion of hydrogen. Meanwhile, the carbon C rushes to combine with ambient oxygen to form CO2. Admit that it's a shame to have a completely clean gas if, to produce it, we emit so much CO2!
A more virtuous possibility is the production of "green" hydrogen by electrolysis: all you need to do is break a water molecule (H2O) and recover oxygen O on one side and dihydrogen H2 on the other. Electrolysis requires electricity, itself provided by nuclear power plants or decarbonized sources. Hence a source called "green." Unfortunately, the energy balance is now disappointing, since you need to provide energy to produce the consumable whose purpose is to provide us with... energy.
The last color in the spectrum is so-called "white" hydrogen, which is found naturally in deposits. But as we have seen, the latter is too volatile to be captured in the atmosphere. Additionally, H2 is also very reactive, burns easily, and the price to pay for such hyperactivity is that it combines very quickly with the elements it encounters as soon as it's produced, unless... Unless it's found 1,000 meters underground in the Lorraine soil where a river of hot water flows. And that, by chance, the H2O molecules flow over rock rich in iron carbonate. A complex reaction called oxidation-reduction then occurs, which breaks down the water molecule into hydrogen and oxygen. And all for free, without needing to resort to a nuclear reactor! Pure H2 gas is therefore continuously produced in a real hydrogen factory operating at full capacity under the feet of the people of Metz. And it's the biggest one on the planet!
La Française d'Energie (FDE), an energy production company with a reduced carbon footprint, has taken hold of the subject, and has the firm intention to develop the enormous perspectives that will follow this major discovery. It is now estimated that this pocket of gas could contain no less than 260 million tonnes of pure hydrogen! The slogan "In France, we don't have oil, but we have ideas" could very soon be transformed into "In France, we have hydrogen in addition to ideas."

